The bacterial reverse mutation test, commonly known as the Ames assay, TOBA-505 - Getting issue details... STATUS uses amino acid-requiring strains of Salmonella typhimurium (S. typhimurium) and Escherichia coli (E. coli) to detect point mutations, which involve substitution, addition, or deletion of 1 or a few DNA base pairs. The principle of this bacterial reverse-mutation test is that it detects chemicals that induce mutations which revert mutations present in the tester strains and restore the functional capability of the bacteria to synthesize an essential amino acid. The revertant bacteria are detected by their ability to grow in the absence of the amino acid required by the parent tester strain.
Example
This example Trial Summary (TS) dataset, shows many informational fields that provide context at the study level.
Dataset Wrapper Debug Message
Please add a row column to your dataset.
Note that there are three trial set parameters that link to other important datasets: SPTOBID, APDEVID, and SMKRGM.
- SPTOBID (Applicant-Defined Tobacco Product ID) is used to uniquely identify the tobacco product. The value of SPTOBID (e.g., CIG01a) matches the value for SPTOBID in all the TOPARMCD-TOVAL pairs in the Tobacco Product Identifiers and Descriptors (TO) dataset example in Section 3.1.2, Product Design Parameters and Conformance Testing, Example 1. The TOPARMCD-TOVAL pairs identify this unique product, CIG01a. The TO domain is described in Section 2.8.8.1, Tobacco Product Identifiers and Descriptors (TO).
- The value of APDEVID (e.g., PUFFMASTER3K) matches the value of SPDEVID in all the DIPARMCD-DIVAL pairs that identify this unique device in the DI dataset. SPDEVID is the applicant-defined device identifier that is used to uniquely identify the device in the Unique Device Identification (DI) dataset (see also Section 2.8.9.7, SEND Device Identifiers (DI)). This is shown in the DI dataset in Section 3.1.3.2, HPHCs, Other Constituents, and Smoking/Vaping Regimens, Example 1.
- SMKRGM serves as a link to the Device-In Use Properties (DU) domain (see also Section 2.8.9.8, SEND Device-In-Use (DU)), where a matching value of SMKRGM indicates parameters of the smoking regimen performed by the smoking machine, as in the DU dataset in Section 3.1.3.2, HPHCs, Other Constituents, and Smoking/Vaping Regimens, Example 2.
Dataset Wrapper Debug Message
Please add a row column to your dataset.
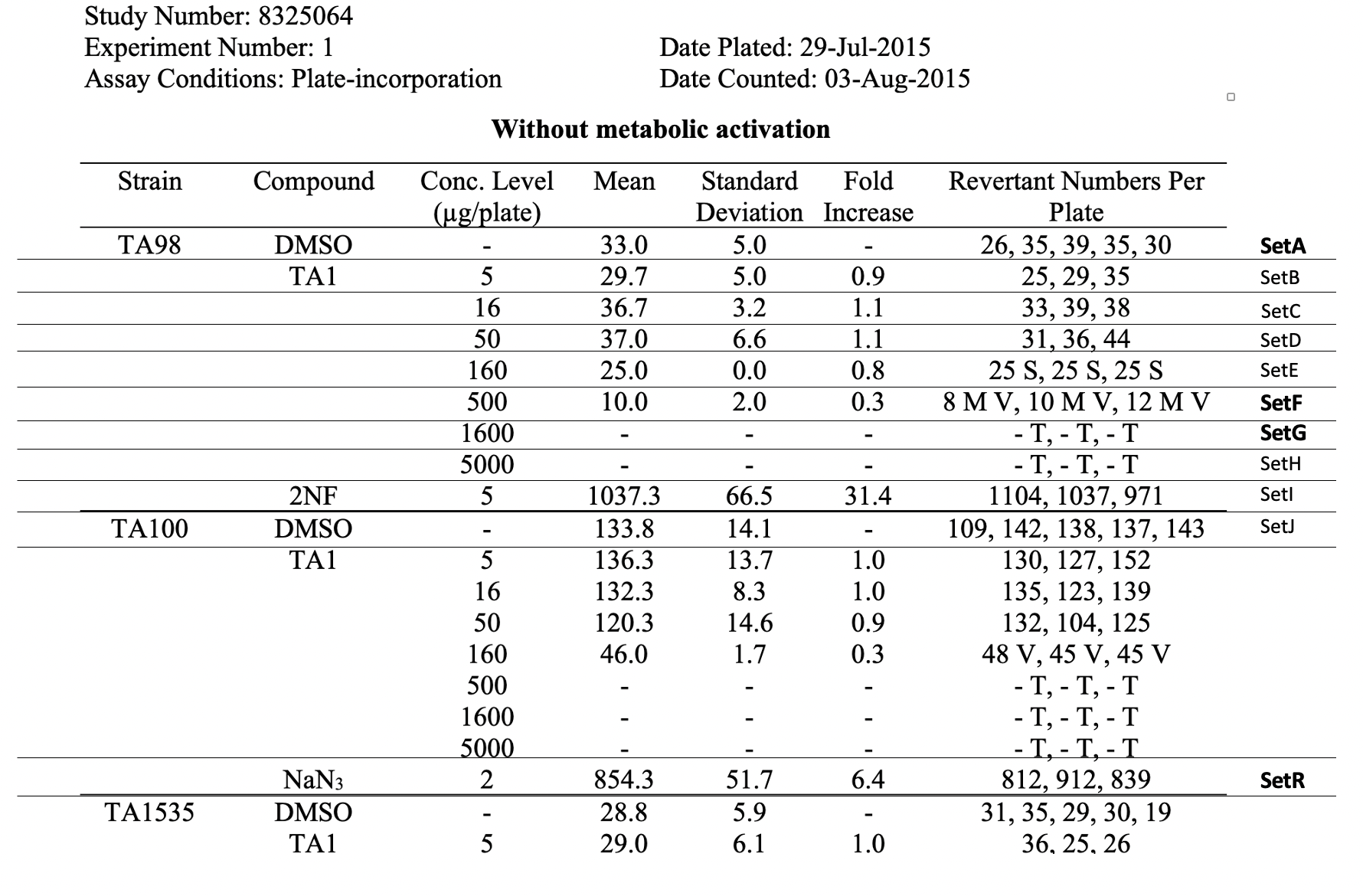
This picture does not depict the more complicated procedures, trial sets, or observational units for the example study. For study 8325064, the applicant chose to define the REFIDs at the trial set level with a single character (e.g., A, F, G, R) and chose to define REFIDs at the observational level based on the location of each tube in 1 of multiple exposure/incubation plates (e.g, 6_1 for plate 6, position 1).
Dataset Wrapper Debug Message
Please add a row column to your dataset.
Dataset Wrapper Debug Message
Please add a row column to your dataset.