Model Documentation
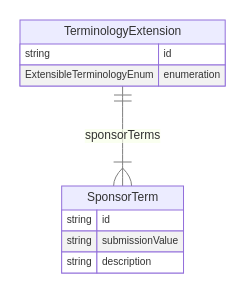
Class: TerminologyExtension
The TerminologyExtension class may be used to define sponsor-defined extensions to extensible ARS terminology. In the ARS model, controlled terminology is implemented through the use of enumerations, each of which specifies a static set of valid values. It is not possible for sponsors to modify enumerations to add additional values but there are 4 "extensible" enumerations where a sponsor-defined term may be used instead of one of the terms in the enumeration:
| Enumeration Name | Enumeration Description | Used In |
|---|---|---|
| AnalysisReasonEnum | The rationale for performing this analysis. It indicates when the analysis was planned. | Analysis |
| AnalysisPurposeEnum | The purpose of the analysis within the body of evidence (e.g., section in the clinical study report). | Analysis |
| OperationRoleEnum | The role that the referenced operation's result plays in the calculation of the result of this operation. | Operation |
| OutputFileTypeEnum | The file format of the file containing output from analyses. | Output |
In situations where a sponsor needs to use a sponsor-defined term instead of one of the defined set of controlled terms, a terminology extension is created. A terminology extension is a set of one or more sponsor-defined terms created to be used instead of the controlled terms defined for one of the "extensible" enumerations.
When needed, a single terminology extension may be created for any of the 4 extensible enumerations, and each terminology extension may contain one or more sponsor terms. The enumeration attribute of the terminology extension indicates the name of the enumeration for which the terminology extension is being created, an identifier value for the terminology extension is specified in the id attribute and one or more sponsor terms are defined in the sponsorTerms attribute, each as an instance of the SponsorTerm class. Each sponsor term has the value to be used when represented in a submission (in the submissionValue attribute), corresponding descriptive text (in the description attribute) and an assigned identifier value (in the id attribute).
The enumerations defined in the ARS model are considered to be CDISC controlled terminology so existing guidance should be followed when assigning sponsor terms for extensible enumerations. This includes, for example, guidance indicating that sponsor terms should not be synonyms for existing controlled terms.