Updates for SDTMIG 4.0
Make sure to update IS assumption #6: Measurements of cytokines, chemokines, and complement proteins should be represented in the Laboratory Test Results (LB) domain.
We need to draft a language to describe when to use IS vs LB for cytokine tests. See notes #3 below.
We recently received this request: https://loinc.org/95971-8/ --- SARS CoV-2 stimulated gamma interferon [Presence] in Blood. We also received requests for the following LOINC codes: 95974-2, 95972-6, and 95973-4. Upon further research on all 4 LOINC codes, they are referring to the IFNg Release Assay, which has been modeled in the LB domain in both TB TAUGs V1 and V2.
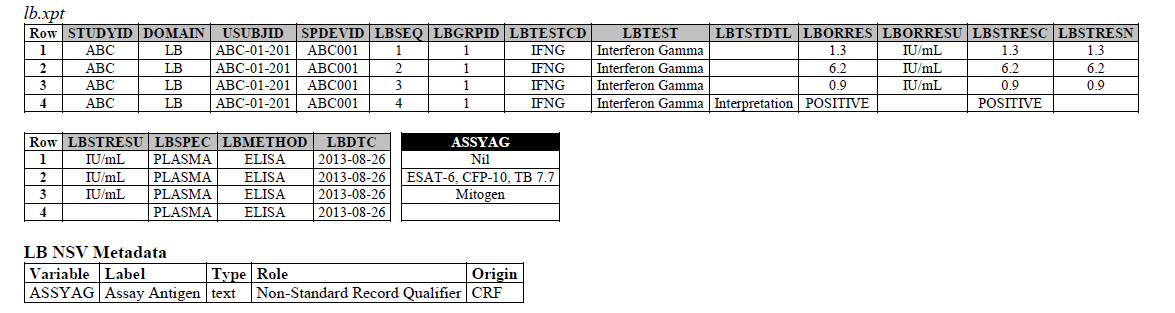
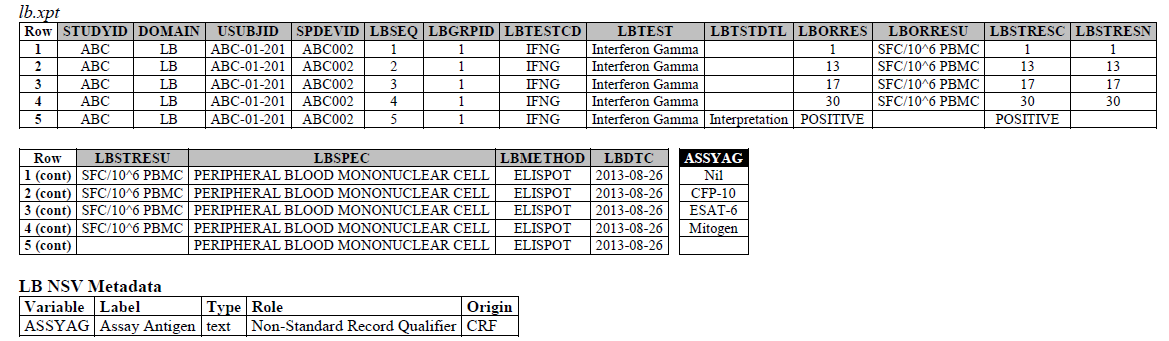
TB-TAUG v2.0 modeling looks like the following:
ELISA:
ELISPOT:
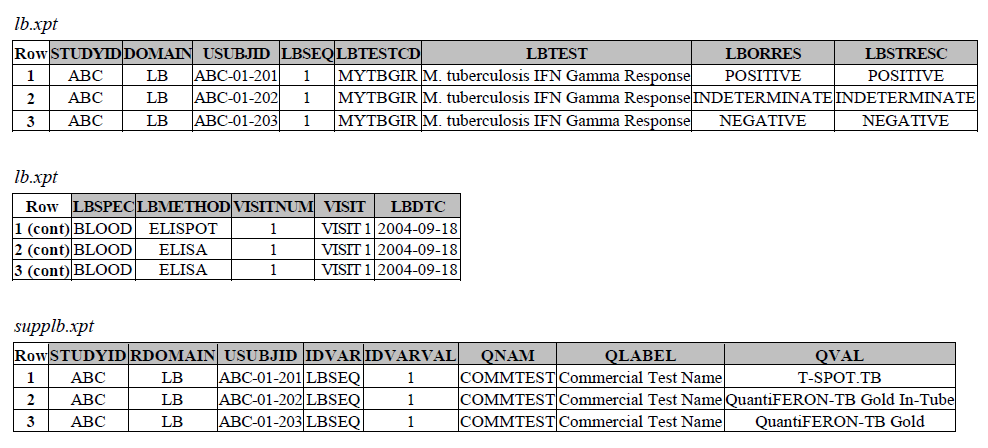
TB-TAUG V1 Modeling looks like the following:
There are several concerns with this modeling:
- There are two ways of modeling Interferon Gamma Release Assay based on TB-TAUG V1 and V2, does V2 modeling replace V1 modeling? Are they modeling the same thing?
- Where do you show that this challenge assay is done against M. Tuberculosis (or any microbe of interest, like SARS-CoV-2), where do you map M. Tb? - NHOID is NOT appropriate and should NOT be used here because in this case, the subject of study is NOT the Mycobacterium tuberculosis itself but whether someone has been previously exposed to M. Tuberculosis, by examining whether there is heightened Interferon-gamma activation. A positive result does not mean the person is currently infected with Mycobacterium tuberculosis, it may mean that the person had been previously infected by, or vaccinated against M. Tuberculosis. NHOID is used when you know the microorganism or a reference strain is present in the testing sample. M. Tuberculosis is mapped to ISCNDAGT to indicate that the stimulating agents are TB antigens.
- Lastly, Interferon-Gamma Release Assay (IGRA) is a classic immunological test, is it correct to map this to the LB domain as per the TB TAUGs? What recommendations do we give to our users in the future when we see a request such as: SARS-CoV-2 stimulated gamma interferon, https://loinc.org/95971-8/
- Model in IS, if you need to report changes in the actual level of IFN-g or number of IFN-g secreting cells, the stimulating and control agents used in the assay, and a "summary interpretation" result of positive, negative, or indeterminate, etc. In this case, you are testing and recording the explicit changes in the IFN-g release immune response, and this belong in the IS domain.
- Model in MB, if the IGRA test is used to indicate whether a subject has ongoing or prior infection of a specific pathogen. In this case, you are doing a microbial detection test based on the interpretation of the IFN-g release immune response. You do NOT care or the lab does not provide you with actual results of the IFN-g related changes.
- The ELISPOT LB example in the TB-TAUG V2 has an error, the unit is reported in SFC/10^6 PBMC so you are counting the number of cells that secrete IFN-g. The example had a LBTEST value of interferon gamma, this is wrong, and should be changed to "Interferon gamma-secreting Cells".
TB TAUG V1 Remodel in the IS domain
T-Cell ELISPOT:
TB TAUG V2 Remodel in the MB domain
The IGRA test is used to indicate whether a subject has ongoing or prior infection of a specific pathogen. In this case, you are doing a microbial detection test based on the interpretation of the IFN-g release immune response. You do NOT care or the lab does not provide you with actual results of the IFN-g related changes.
MBTEST = M. tuberculosis IFN Gamma Response (C92241), this test will be deprecated.