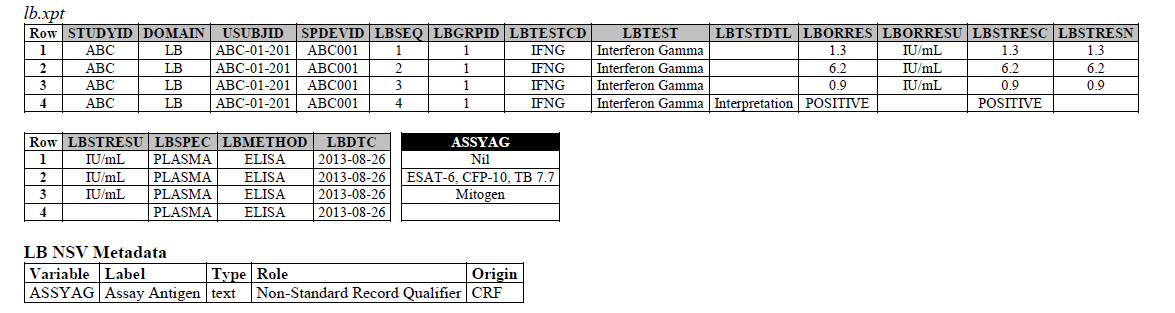
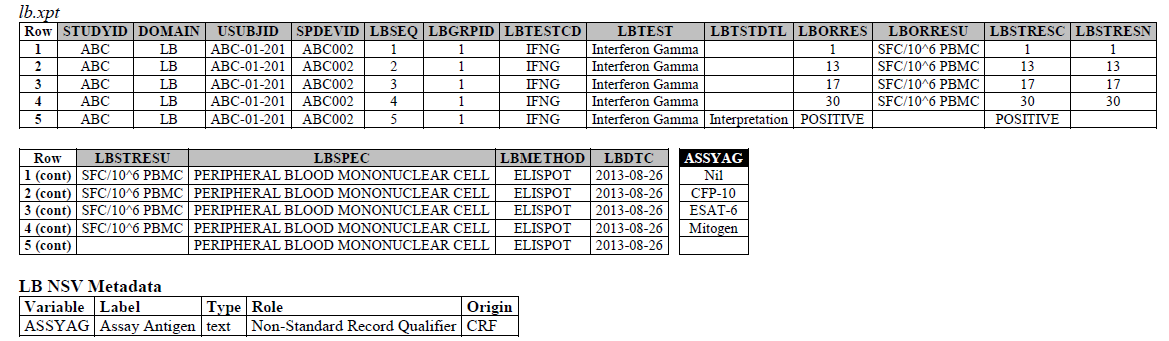
We recently received this request: https://loinc.org/95971-8/ --- SARS CoV-2 stimulated gamma interferon [Presence] in Blood. We also recieved requests for the following LOINC codes: 95974-2, 95972-6, and 95973-4. Upon further research on all 4 LOINC codes, they are referring to the IFNg Response Assay, which has been modeled in the LB domain in the TB-TAUG.
The IFNG Response test toward M. Tuberculosis is modeled in the LB domain, in the TB-TAUG as the following:
ELISA:
ELISPOT:
There are several issues with this modeling:
- Where do you show that this challenge assay is done against M. Tuberculosis (or any microbe of interest, like SARS-CoV-2)? - use NHOID? FOLLOW-UP WITH TEAM
- CP domain has developed a new standard variable called Test Condition Agent/CNDAGT (CP Specification), which is used to represent stimulating agents, like the values in ASSYAG above, should this variable be added to LB, or IS? FOLLOW-UP WITH TEAM
- Lastly, Interferon-Gamma Response Assay is a classic immunological test, is it correct to map this to the LB domain?
- Can we model this in the IS domain, see example dataset below. Yes - TEAM AGREES
- If we model this test in IS, how do we distinguish it from the INFg test in lab. Can we draw the line where if we are running a routine lab test looking for IFNg levels it goes to LB vs. IFNg challenge/response test toward a specific, known microorganism then it goes to IS? Yes-TEAM AGREES
If we were to model this in IS:
Similarly if we were to model Jozef's request in IS for https://loinc.org/95971-8/ --- SARS CoV-2 stimulated gamma interferon [Presence] in Blood