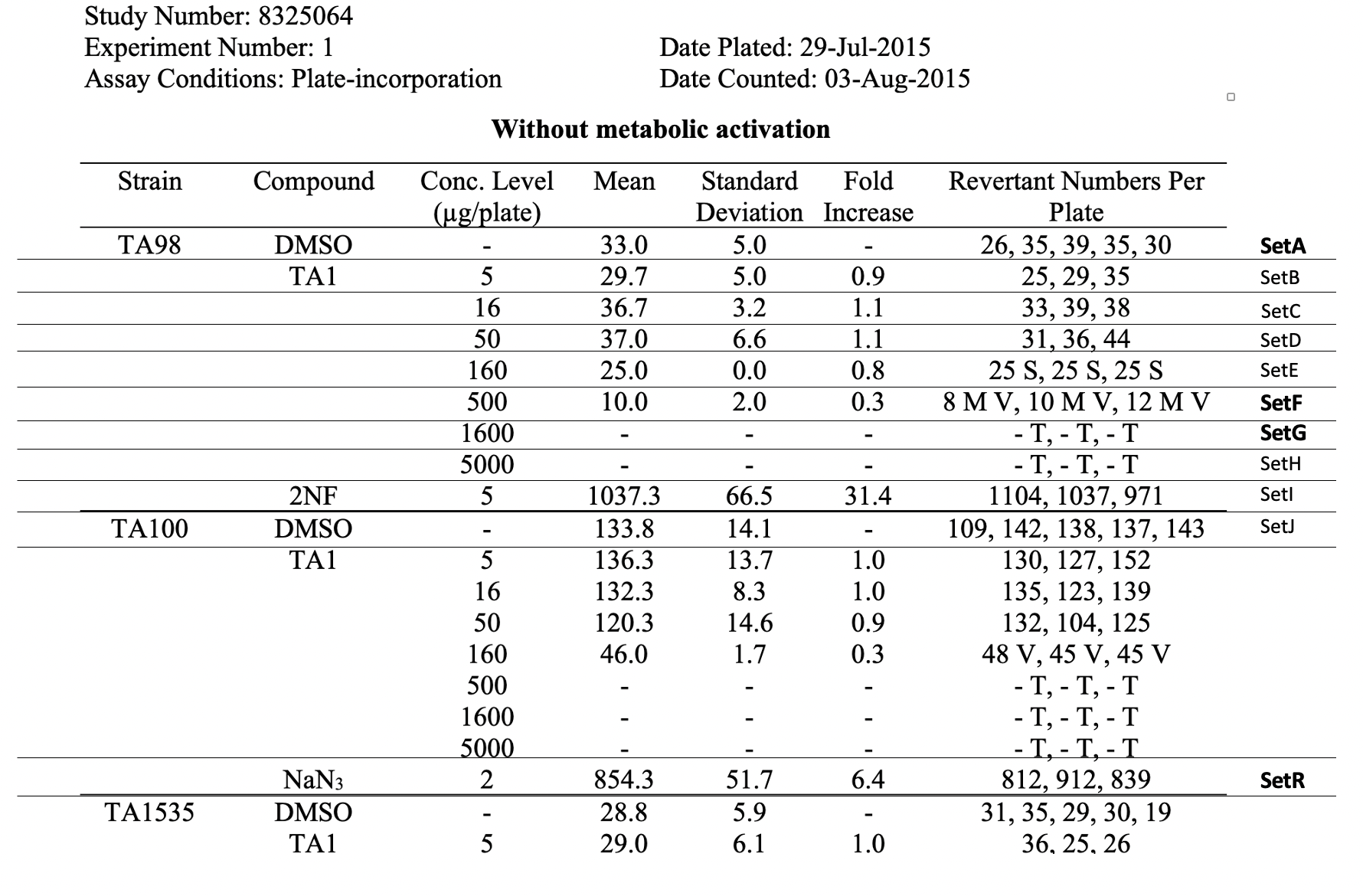
This is an example showing the report table, trial design, and results from a study's in vitro bacterial reverse mutation test TOBA-774 - Getting issue details... STATUS TOBA-505 - Getting issue details... STATUS using 4 different amino acid-requiring strains of S. typhimurium and 1 strain of E. coli to detect point mutations. TOBA-129 - Getting issue details... STATUS
This example Trial Summary (TS) dataset TOBA-481 - Getting issue details... STATUS , TOBA-368 - Getting issue details... STATUS shows many informational fields TOBA-369 - Getting issue details... STATUS that provide context at the study level.
TOBA-663 - Getting issue details... STATUS TOBA-664 - Getting issue details... STATUS
Dataset Wrapper Debug Message
Row captions have been provided, but there doesn't seem to be a dataset to which the row captions would apply.
| Rows 1-14: | Show trial set SetSTM, with trial set parameters and trial set values that comprise the test conditions for all data for the species S. typhimurium. |
|---|---|
| Rows 15, 24, 33, 42: | Show that SetA, SetF, SetG, and SetR each have a parent set code of SetSTM. As a child set of SetSTM, each of these sets inherit the test conditions of SetSTM. |
| Rows 15-23: | Show trial set parameters and trial set values that comprise the test conditions for the set SetA. SetA is the data for the vehicle control (i.e., Strain of TA98, vehicle control with concentration value of 0). SetA is associated with the first row, labeled "SetA", in the report table for example study 8325064. |
| Rows 24-32: | Show trial set parameters and trial set values that comprise the test conditions for the set SetF. SetF is the data for the strain of TA98 with a concentration value of 500 µg/plate. SetF is associated with the sixth row, labeled "SetF", in the report table for example study 8325064. |
| Rows 33-41: | Show trial set parameters and trial set values that comprise the test conditions for the set SetG. SetG is the data for the strain of TA98 with a concentration value of 1600 µg/plate. SetG is associated with the seventh row, labeled "SetG", in the report table for example study 8325064. |
| Rows 42-50: | Show trial set parameters and trial set values that comprise the test conditions for the set SetR. SetR is the data for the strain of TA100, positive control with a concentration value of 2 µg/plate. SetR is associated with the eighteenth row, labeled "SetR", in the report table for example study 8325064. |
| Row 10: | SPTOBID (Applicant-Defined Tobacco Product ID) is used to uniquely identify the tobacco product. The value of SPTOBID, in this case CIG01a, matches the value for SPTOBID in all the TOPARMCD-TOVAL pairs in the Tobacco Product Identifiers and Descriptors (TO) dataset example in Section 3.1.2, Product Design Parameters and Conformance Testing, Example 1. The TOPARMCD-TOVAL pairs identify this unique product, CIG01a. The TO domain is described in Section 2.8.8.1, Tobacco Product Identifiers and Descriptors (TO). |
| Row 13: | SPDEVID is the applicant-defined device identifier that is used to uniquely identify the device in the Unique Device Identification (DI) dataset (see also Section 2.8.9.7, SEND Device Identifiers (DI)). The value of SPDEVID, in this case PUFFMASTER3K, matches the value of SPDEVID in all the DIPARMCD-DIVAL pairs that identify this unique device in the DI dataset. This is shown in the DI dataset in Section 3.1.3.2, HPHCs, Other Constituents, and Smoking/Vaping Regimens, Example 1. |
| Row 14: | Shows a value for SMKRGM. SMKRGM serves as a link to the Device-In Use Properties (DU) domain (see also Section 2.8.9.8, SEND Device-In-Use (DU)), where a matching value of SMKRGM indicates parameters of the smoking regimen performed by the smoking machine, as in the DU dataset in Section 3.1.3.2, HPHCs, Other Constituents, and Smoking/Vaping Regimens, Example 2. |
Unable to render dataset table
Please place only one table inside the macro.
THIS (RED FONT) IS THE OLD DATASET PRIOR TO "flattening":
| STUDYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL | |
|---|---|---|---|---|---|---|---|---|
| 1 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 1 | SPECIES | Species | SALMONELLA TYPHIMURIUM |
| 2 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 2 | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 |
| 3 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 3 | IVTDTRG | In vitro Treatment Duration Target | 72 |
| 4 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 4 | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 |
| 5 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 5 | IVTDU | In vitro TreatmentRowDuration Unit | HOURS |
| 6 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 6 | INCBTMP | Incubation Temperature | 37 |
| 7 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 7 | INCBTMPU | Incubation Temperature Unit | C |
| 8 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 8 | ATMRHP | Atmospheric Relative Humidity Percent | 50 |
| 9 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 9 | ATMCO2P | Atmospheric CO2 Percent | 5 |
| 10 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 10 | SPTOBID | Applicant-defined tobacco identifier | CIG01a |
| 11 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 11 | EXPTYP | Exposure Type | Submerged |
| 12 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 12 | SAMTYP | Sample Type | Total Particulate Matter in PBS |
| 13 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 13 | SPDEVID | Applicant-defined device identifier | PUFFMASTER3K |
| 14 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 14 | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN |
| 15 | 8325064 | TX | SetA | A-TA98-C0 | 15 | PSETCD | Parent Set Code | SetSTM |
| 16 | 8325064 | TX | SetA | A-TA98-C0 | 15 | STRAIN | Strain/Substrain | TA98 |
| 17 | 8325064 | TX | SetA | A-TA98-C0 | 16 | METACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
| 18 | 8325064 | TX | SetA | A-TA98-C0 | 17 | METACTFL | Presence of Metabolic Activation Flag | N |
| 19 | 8325064 | TX | SetA | A-TA98-C0 | 18 | ITVNAM | Intervention Article Name | DMSO |
| 20 | 8325064 | TX | SetA | A-TA98-C0 | 19 | ITVTYPE | Intervention Article Type | VEHICLE |
| 21 | 8325064 | TX | SetA | A-TA98-C0 | 20 | ITVCONC | Intervention Article Concentration | 100 |
| 22 | 8325064 | TX | SetA | A-TA98-C0 | 21 | ITVCONCU | Intervention Article Concentration Unit | % |
| 23 | 8325064 | TX | SetA | A-TA98-C0 | 22 | TRTV | Treatment Vehicle | DMSO |
| 24 | 8325064 | TX | SetF | F-TA98-C500 TOBA-485 - Getting issue details... STATUS | 23 | PSETCD | Parent Set Code | SetSTM |
| 25 | 8325064 | TX | SetF | F-TA98-C500 | 24 | STRAIN | Strain/Substrain | TA98 |
| 26 | 8325064 | TX | SetF | F-TA98-C500 | 25 | METACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
| 27 | 8325064 | TX | SetF | F-TA98-C500 | 26 | METACTFL | Presence of Metabolic Activation Flag | N |
| 28 | 8325064 | TX | SetF | F-TA98-C500 | 27 | ITVNAM | Intervention Article Name | TA1 |
| 29 | 8325064 | TX | SetF | F-TA98-C500 | 28 | ITVTYPE | Intervention Article Type | PRODUCT |
| 30 | 8325064 | TX | SetF | F-TA98-C500 | 29 | ITVCONC | Intervention Article Concentration | 500 |
| 31 | 8325064 | TX | SetF | F-TA98-C500 | 30 | ITVCONCU | Intervention Article Concentration Unit | ug/plate |
| 32 | 8325064 | TX | SetF | F-TA98-C500 | 31 | TRTV | Treatment Vehicle | DMSO |
| 33 | 8325064 | TX | SetG | G-TA98-C1600 | 31 | PSETCD | Parent Set Code | SetSTM |
| 34 | 8325064 | TX | SetG | G-TA98-C1600 | 32 | STRAIN | Strain/Substrain | TA98 |
| 35 | 8325064 | TX | SetG | G-TA98-C1600 | 33 | METACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
| 36 | 8325064 | TX | SetG | G-TA98-C1600 | 34 | METACTFL | Presence of Metabolic Activation Flag | N |
| 37 | 8325064 | TX | SetG | G-TA98-C1600 | 35 | ITVNAM | Intervention Article Name | TA1 |
| 38 | 8325064 | TX | SetG | G-TA98-C1600 | 36 | ITVTYPE | Intervention Article Type | Product |
| 39 | 8325064 | TX | SetG | G-TA98-C1600 | 37 | ITVCONC | Intervention Article Concentration | 1600 |
| 40 | 8325064 | TX | SetG | G-TA98-C1600 | 38 | ITVCONCU | Intervention Article Concentration Unit | ug/plate |
| 41 | 8325064 | TX | SetG | G-TA98-C1600 | 39 | TRTV | Treatment Vehicle | DMSO |
| 42 | 8325064 | TX | SetR | R-TA100-C2 | 39 | PSETCD | Parent Set Code | SetSTM |
| 43 | 8325064 | TX | SetR | R-TA100-C2 | 40 | STRAIN | Strain/Substrain | TA100 |
| 44 | 8325064 | TX | SetR | R-TA100-C2 | 41 | METACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
| 45 | 8325064 | TX | SetR | R-TA100-C2 | 42 | METACTFL | Presence of Metabolic Activation Flag | N |
| 46 | 8325064 | TX | SetR | R-TA100-C2 | 43 | ITVNAM | Intervention Article Name | NaN3 |
| 47 | 8325064 | TX | SetR | R-TA100-C2 | 44 | ITVTYPE | Intervention Article Type | Positive Control |
| 48 | 8325064 | TX | SetR | R-TA100-C2 | 45 | ITVCONC | Intervention Article Concentration | 2 |
| 49 | 8325064 | TX | SetR | R-TA100-C2 | 46 | ITVCONCU | Intervention Article Concentration Unit | ug/plate |
| 50 | 8325064 | TX | SetR | R-TA100-C2 | 47 | TRTV | Treatment Vehicle | DMSO |
THIS IS A DRAFTED MOCK-UP OF THE "flattened" version of TX dataset:
| STUDYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL | |
|---|---|---|---|---|---|---|---|---|
| 1 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 1 | SPECIES | Species | SALMONELLA TYPHIMURIUM |
| 2 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 2 | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 |
| 3 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 3 | IVTDTRG | In vitro Treatment Duration Target | 72 |
| 4 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 4 | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 |
| 5 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 5 | IVTDU | In vitro TreatmentRowDuration Unit | HOURS |
| 6 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 6 | INCBTMP | Incubation Temperature | 37 |
| 7 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 7 | INCBTMPU | Incubation Temperature Unit | C |
| 8 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 8 | ATMRHP | Atmospheric Relative Humidity Percent | 50 |
| 9 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 9 | ATMCO2P | Atmospheric CO2 Percent | 5 |
| 10 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 10 | SPTOBID | Applicant-defined tobacco identifier | CIG01a |
| 11 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 11 | EXPTYP | Exposure Type | Submerged |
| 12 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 12 | SAMTYP | Sample Type | Total Particulate Matter in PBS |
| 13 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 13 | SPDEVID | Applicant-defined device identifier | PUFFMASTER3K |
| 14 | 8325064 | TX | SetSTM | SALMONELLA TYPHIMURIUM | 14 | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN |
| 15 | 8325064 | TX | SetA | A-TA98-C0 | 1 | SPECIES | Species | SALMONELLA TYPHIMURIUM |
| 16 | 8325064 | TX | SetA | A-TA98-C0 | 2 | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 |
| 17 | 8325064 | TX | SetA | A-TA98-C0 | 3 | IVTDTRG | In vitro Treatment Duration Target | 72 |
| 18 | 8325064 | TX | SetA | A-TA98-C0 | 4 | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 |
| 19 | 8325064 | TX | SetA | A-TA98-C0 | 5 | IVTDU | In vitro TreatmentRowDuration Unit | HOURS |
| 20 | 8325064 | TX | SetA | A-TA98-C0 | 6 | INCBTMP | Incubation Temperature | 37 |
| 21 | 8325064 | TX | SetA | A-TA98-C0 | 7 | INCBTMPU | Incubation Temperature Unit | C |
| 22 | 8325064 | TX | SetA | A-TA98-C0 | 8 | ATMRHP | Atmospheric Relative Humidity Percent | 50 |
| 23 | 8325064 | TX | SetA | A-TA98-C0 | 9 | ATMCO2P | Atmospheric CO2 Percent | 5 |
| 24 | 8325064 | TX | SetA | A-TA98-C0 | 10 | SPTOBID | Applicant-defined tobacco identifier | CIG01a |
| 25 | 8325064 | TX | SetA | A-TA98-C0 | 11 | EXPTYP | Exposure Type | Submerged |
| 26 | 8325064 | TX | SetA | A-TA98-C0 | 12 | SAMTYP | Sample Type | Total Particulate Matter in PBS |
| 27 | 8325064 | TX | SetA | A-TA98-C0 | 13 | SPDEVID | Applicant-defined device identifier | PUFFMASTER3K |
| 28 | 8325064 | TX | SetA | A-TA98-C0 | 14 | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN |
| 29 | 8325064 | TX | SetA | A-TA98-C0 | 15 | PSETCD | Parent Set Code | SetSTM |
| 30 | 8325064 | TX | SetA | A-TA98-C0 | 15 | STRAIN | Strain/Substrain | TA98 |
| 31 | 8325064 | TX | SetA | A-TA98-C0 | 16 | METACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
| 32 | 8325064 | TX | SetA | A-TA98-C0 | 17 | METACTFL | Presence of Metabolic Activation Flag | N |
| 33 | 8325064 | TX | SetA | A-TA98-C0 | 18 | ITVNAM | Intervention Article Name | DMSO |
| 34 | 8325064 | TX | SetA | A-TA98-C0 | 19 | ITVTYPE | Intervention Article Type | VEHICLE |
| 35 | 8325064 | TX | SetA | A-TA98-C0 | 20 | ITVCONC | Intervention Article Concentration | 100 |
| 36 | 8325064 | TX | SetA | A-TA98-C0 | 21 | ITVCONCU | Intervention Article Concentration Unit | % |
| 37 | 8325064 | TX | SetA | A-TA98-C0 | 22 | TRTV | Treatment Vehicle | DMSO |
| 38 | 8325064 | TX | SetF | F-TA98-C500 TOBA-485 - Getting issue details... STATUS | 23 | PSETCD | Parent Set Code | SetSTM |
| 39 | 8325064 | TX | SetF | F-TA98-C500 | 24 | STRAIN | Strain/Substrain | TA98 |
| 40 | 8325064 | TX | SetF | F-TA98-C500 | 25 | METACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
| 41 | 8325064 | TX | SetF | F-TA98-C500 | 26 | METACTFL | Presence of Metabolic Activation Flag | N |
| 42 | 8325064 | TX | SetF | F-TA98-C500 | 27 | ITVNAM | Intervention Article Name | TA1 |
| 43 | 8325064 | TX | SetF | F-TA98-C500 | 28 | ITVTYPE | Intervention Article Type | PRODUCT |
| 44 | 8325064 | TX | SetF | F-TA98-C500 | 29 | ITVCONC | Intervention Article Concentration | 500 |
| 45 | 8325064 | TX | SetF | F-TA98-C500 | 30 | ITVCONCU | Intervention Article Concentration Unit | ug/plate |
| 46 | 8325064 | TX | SetF | F-TA98-C500 | 31 | TRTV | Treatment Vehicle | DMSO |
| 47 | 8325064 | TX | SetG | G-TA98-C1600 | 31 | PSETCD | Parent Set Code | SetSTM |
| 48 | 8325064 | TX | SetG | G-TA98-C1600 | 32 | STRAIN | Strain/Substrain | TA98 |
| 49 | 8325064 | TX | SetG | G-TA98-C1600 | 33 | METACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
| 50 | 8325064 | TX | SetG | G-TA98-C1600 | 34 | METACTFL | Presence of Metabolic Activation Flag | N |
| 51 | 8325064 | TX | SetG | G-TA98-C1600 | 35 | ITVNAM | Intervention Article Name | TA1 |
| 52 | 8325064 | TX | SetG | G-TA98-C1600 | 36 | ITVTYPE | Intervention Article Type | Product |
| 53 | 8325064 | TX | SetG | G-TA98-C1600 | 37 | ITVCONC | Intervention Article Concentration | 1600 |
| 54 | 8325064 | TX | SetG | G-TA98-C1600 | 38 | ITVCONCU | Intervention Article Concentration Unit | ug/plate |
| 55 | 8325064 | TX | SetG | G-TA98-C1600 | 39 | TRTV | Treatment Vehicle | DMSO |
| 56 | 8325064 | TX | SetR | R-TA100-C2 | 39 | PSETCD | Parent Set Code | SetSTM |
| 57 | 8325064 | TX | SetR | R-TA100-C2 | 40 | STRAIN | Strain/Substrain | TA100 |
| 58 | 8325064 | TX | SetR | R-TA100-C2 | 41 | METACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
| 59 | 8325064 | TX | SetR | R-TA100-C2 | 42 | METACTFL | Presence of Metabolic Activation Flag | N |
| 60 | 8325064 | TX | SetR | R-TA100-C2 | 43 | ITVNAM | Intervention Article Name | NaN3 |
| 61 | 8325064 | TX | SetR | R-TA100-C2 | 44 | ITVTYPE | Intervention Article Type | Positive Control |
| 62 | 8325064 | TX | SetR | R-TA100-C2 | 45 | ITVCONC | Intervention Article Concentration | 2 |
| 63 | 8325064 | TX | SetR | R-TA100-C2 | 46 | ITVCONCU | Intervention Article Concentration Unit | ug/plate |
| 64 | 8325064 | TX | SetR | R-TA100-C2 | 47 | TRTV | Treatment Vehicle | DMSO |
This picture does not depict the more complicated procedures, TOBA-370 - Getting issue details... STATUS trial sets, or observational units for the example study. For study 8325064, the applicant chose to define the REFIDs at the trial set level with a single character (e.g., A, F, G, R) and chose to define REFIDs at the observational level based on the location of each tube in 1 of multiple exposure/incubation plates (e.g, 6_1 for plate 6, position 1).
Dataset Wrapper Debug Message
Row captions have been provided, but there doesn't seem to be a dataset to which the row captions would apply.
| Row 1: | Shows the value of REFID=STM. This REFID refers to the trial set with a SETCD of "SetSTM", as defined in the TX dataset. LEVEL=1 and LVLDESC="TRIAL SET" indicates this identifier relates to the entire trial set. |
|---|---|
| Rows 1, 2, 8, 12, 16: | Show that the trial set SetSTM is the parent of set codes SetA, SetF, SetG, and SetR. |
| Row 2: | Shows the value of REFID=A. This REFID refers to the trial set with a SETCD of "SetA", as defined in the TX dataset. LEVEL=2 and LVLDESC="EXPERIMENTAL UNIT/TRIAL SET". TRIAL SET indicates this identifier relates to the entire trial set. EXPERIMENTAL UNIT indicates this identifier received the intervention article, DMSO. |
| Rows 3-7: | Show the values of 5 observational units (1_1 through 1_5) that are within the parent experimental unit, REFID=A. |
| Row 8: | Shows the value of REFID=F. This REFID refers to the trial set with a SETCD of "SetF", as defined in the TX dataset. LEVEL=2 and LVLDESC="EXPERIMENTAL UNIT/TRIAL SET". TRIAL SET indicates this identifier relates to the entire trial set. EXPERIMENTAL UNIT indicates this identifier received the intervention article, TA1. |
| Rows 9-11: | Show the values of 3 observational units (6_1 through 6_3) that are within the parent experimental unit, REFID=F. |
| Row 12: | Shows the value of REFID=G. This REFID refers to the trial set with a SETCD of "SetG", as defined in the TX dataset. LEVEL=2 and LVLDESC="EXPERIMENTAL UNIT/TRIAL SET". TRIAL SET indicates this identifier relates to the entire trial set. EXPERIMENTAL UNIT indicates this identifier received the intervention article, TA1. |
| Rows 13-15: | Show the values of 3 observational units (7_1 through 7_3) that are within the parent experimental unit, REFID=G. |
| Row 16: | Shows the value of REFID=R. This REFID refers to the trial set with a SETCD of "SetG", as defined in the TX dataset. LEVEL=2 and LVLDESC="EXPERIMENTAL UNIT/TRIAL SET". TRIAL SET indicates this identifier relates to the entire trial set. EXPERIMENTAL UNIT indicates this identifier received the intervention article, NaN3. |
| Rows 17-19: | Show the values of 3 observational units (18_1 through 18_3) that are within the parent experimental unit, REFID=R. |
Unable to render dataset table
Please place only one table inside the macro.
OLD TABLE (prior to flattening Parent/Child in TX dataset)
When this table is removed, remove SetSTM from TX.
Row | STUDYID | SETCD | REFID | PARENT | LEVEL | LVLDESC |
|---|---|---|---|---|---|---|
1 | 8325064 | SetSTM | STM | 1 | TRIAL SET | |
2 | 8325064 | SetA | A | STM | 2 | EXPERIMENTAL UNIT/TRIAL SET |
3 | 8325064 | SetA | 1_1 | A | 3 | OBSERVATIONAL UNIT |
4 | 8325064 | SetA | 1_2 | A | 3 | OBSERVATIONAL UNIT |
5 | 8325064 | SetA | 1_3 | A | 3 | OBSERVATIONAL UNIT |
6 | 8325064 | SetA | 1_4 | A | 3 | OBSERVATIONAL UNIT |
7 | 8325064 | SetA | 1_5 | A | 3 | OBSERVATIONAL UNIT |
8 | 8325064 | SetF | F | STM | 2 | EXPERIMENTAL UNIT/TRIAL SET |
9 | 8325064 | SetF | 6_1 | F | 3 | OBSERVATIONAL UNIT |
10 | 8325064 | SetF | 6_2 | F | 3 | OBSERVATIONAL UNIT |
11 | 8325064 | SetF | 6_3 | F | 3 | OBSERVATIONAL UNIT |
12 | 8325064 | SetG | G | STM | 2 | EXPERIMENTAL UNIT/TRIAL SET |
13 | 8325064 | SetG | 7_1 | G | 3 | OBSERVATIONAL UNIT |
| 14 | 8325064 | SetG | 7_2 | G | 3 | OBSERVATIONAL UNIT |
| 15 | 8325064 | SetG | 7_3 | G | 3 | OBSERVATIONAL UNIT |
| 16 | 8325064 | SetG | R | STM | 2 | EXPERIMENTAL UNIT/TRIAL SET |
| 17 | 8325064 | SetR | 18_1 | R | 3 | OBSERVATIONAL UNIT |
| 18 | 8325064 | SetR | 18_2 | R | 3 | OBSERVATIONAL UNIT |
| 19 | 8325064 | SetR | 18_3 | R | 3 | OBSERVATIONAL UNIT |
NEW (remain AFTER we flatten TX dataset)
Row | STUDYID | SETCD | REFID | PARENT | LEVEL | LVLDESC |
|---|---|---|---|---|---|---|
1 | 8325064 | SetA | A | 1 | EXPERIMENTAL UNIT/TRIAL SET | |
2 | 8325064 | SetA | 1_1 | A | 2 | OBSERVATIONAL UNIT |
3 | 8325064 | SetA | 1_2 | A | 2 | OBSERVATIONAL UNIT |
4 | 8325064 | SetA | 1_3 | A | 2 | OBSERVATIONAL UNIT |
5 | 8325064 | SetA | 1_4 | A | 2 | OBSERVATIONAL UNIT |
6 | 8325064 | SetA | 1_5 | A | 2 | OBSERVATIONAL UNIT |
7 | 8325064 | SetF | F | 1 | EXPERIMENTAL UNIT/TRIAL SET | |
8 | 8325064 | SetF | 6_1 | F | 2 | OBSERVATIONAL UNIT |
9 | 8325064 | SetF | 6_2 | F | 2 | OBSERVATIONAL UNIT |
10 | 8325064 | SetF | 6_3 | F | 2 | OBSERVATIONAL UNIT |
11 | 8325064 | SetG | G | 1 | EXPERIMENTAL UNIT/TRIAL SET | |
12 | 8325064 | SetG | 7_1 | G | 2 | OBSERVATIONAL UNIT |
13 | 8325064 | SetG | 7_2 | G | 2 | OBSERVATIONAL UNIT |
| 14 | 8325064 | SetG | 7_3 | G | 2 | OBSERVATIONAL UNIT |
| 15 | 8325064 | SetG | R | 1 | EXPERIMENTAL UNIT/TRIAL SET | |
| 16 | 8325064 | SetR | 18_1 | R | 2 | OBSERVATIONAL UNIT |
| 17 | 8325064 | SetR | 18_2 | R | 2 | OBSERVATIONAL UNIT |
| 18 | 8325064 | SetR | 18_3 | R | 2 | OBSERVATIONAL UNIT |
Dataset Wrapper Debug Message
Please add a row column to your dataset.