- Created by Lou Ann Kramer, last modified on Mar 22, 2023
You are viewing an old version of this page. View the current version.
Compare with Current View Page History
« Previous Version 104 Next »
This example shows a sample report table, trial design and results data for Study #123 for the determination of the in vitro genotoxicity potential of tobacco products in the in vitro Micronucleus Assay.
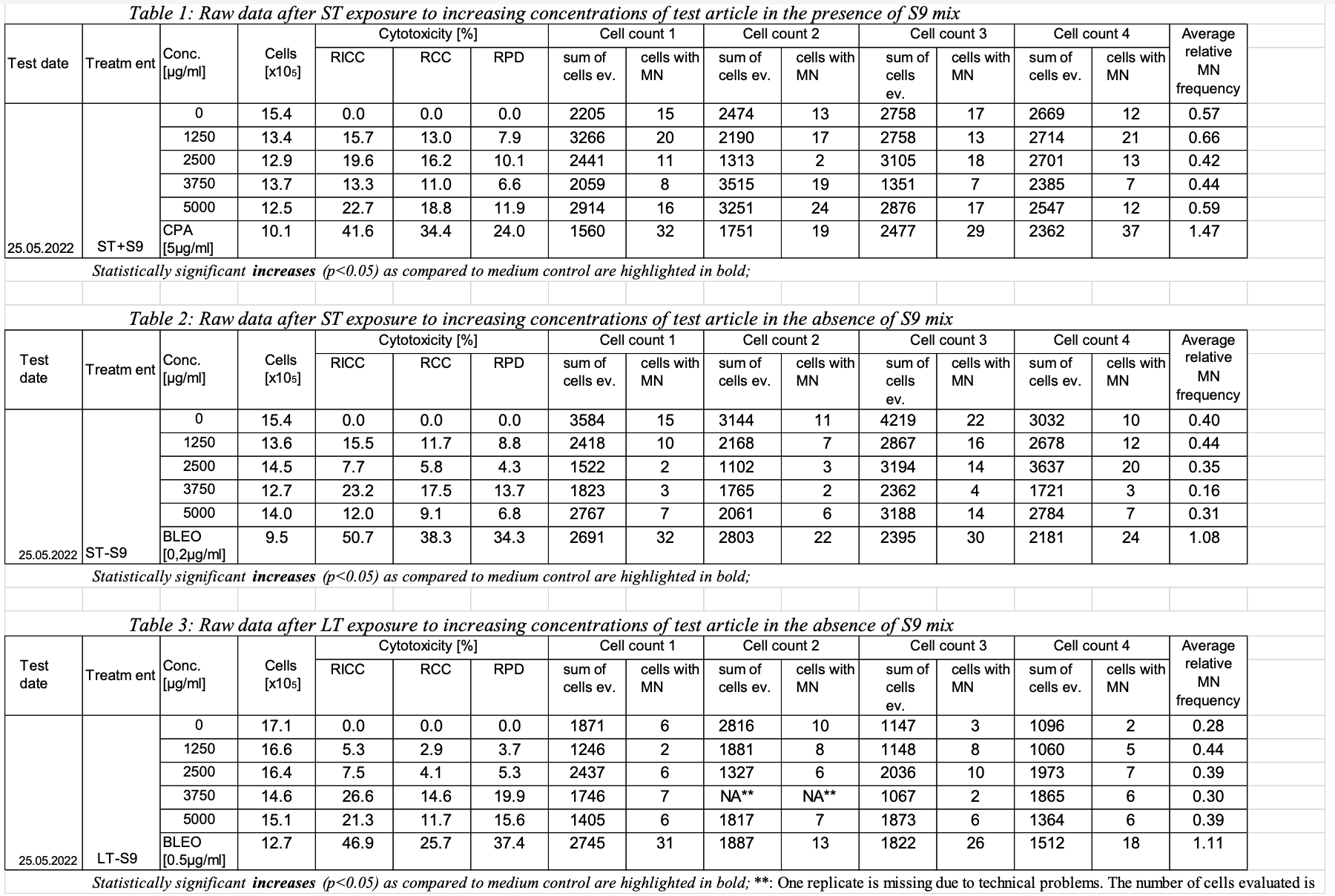
| Rows 1-2: | Show two records for TSPARMCD = "GLPTYP", using TSSEQ to indicate multiple records, since both GLP Types apply for this example study. |
|---|---|
| Row 8: | Shows that the sponsor's study reference ID is not applicable. |
| Rows 9-12: | Show that TSGRPID has been used to link records (name, location, country) related to the test facility (TSGRPID = 1). The Study Director is associated with the Test Facility. |
ts.xpt
xx.xpt
Row | STUDYID | ASSAYID | DOMAIN | TSSEQ | TSGRPID | TSPARMCD | TSPARM | TSVAL | TSVALNF |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 123 | NRU | TS | 1 | GLPTYP | Good Laboratory Practice Type | FDA | ||
| 2 | 123 | NRU | TS | 2 | GLPTYP | Good Laboratory Practice Type | OECD | ||
| 3 | 123 | NRU | TS | 1 | STSTDTC | Study Start Date | 2022-05-25 | ||
| 4 | 123 | NRU | TS | 1 | STITLE | Study Title | Determination of the in vitro genotoxicity potential using the in vitro Neutral Red Uptake assay | ||
| 5 | 123 | NRU | TS | 1 | SNDIGVER | SEND Implementation Guide Version | TOBACCO IMPLEMENTATION GUIDE VERSION 1.0 | ||
| 6 | 123 | NRU | TS | 1 | SNDCTVER | SEND Controlled Terminology Version | SEND Terminology 2021-09-30 | ||
| 7 | 123 | NRU | TS | 1 | SSPONSOR | Sponsor Organization | Example Sponsor Inc. | ||
| 8 | 123 | NRU | TS | 1 | SPREFID | Sponsor Study Reference ID | NOT APPLICABLE | ||
| 9 | 123 | NRU | TS | 1 | 1 | TSTFNAM | Test Facility Name | Example Test Lab Name | |
| 10 | 123 | NRU | TS | 1 | 1 | TSTFLOC | Test Facility Location | 10 Somewhere Street, Montgomery, AL 10000 | |
| 11 | 123 | NRU | TS | 1 | 1 | TFCNTRY | Test Facility Country | USA | |
| 12 | 123 | NRU | TS | 1 | 1 | STDIR | Study Director | Dr. R. Smith | |
| 13 | 123 | NRU | TS | 1 | GLPFL | GLP Flag | Y | ||
| 14 | 123 | NRU | TS | 1 | ASTD | Assay Standard | NIH Publication No. 07-4519 | ||
| 15 | 123 | NRU | TS | 1 | ASTDV | Assay Standard Version | 2006-11 | ||
| 16 | 123 | NRU | TS | 1 | SSTYP | Study Type | GENOTOXICITY IN VITRO | ||
| 17 | 123 | NRU | TS | 1 | SSSTYP | Study Sub Type | In Vitro Neutral Red Uptake | ||
| 18 | 123 | NRU | TS | 1 | SPECIES | Species | Hamster | ||
| 19 | 123 | NRU | TS | 1 | TESTSYS | Test System | CHO |
Dataset Wrapper Debug Message
Please add a row column to your dataset.
| Rows 1-24: | Show trial set parameters and trial set values that comprise the test conditions for trial set A1. Set A1 is the data for the negative control (concentration 0) with short term exposure and metabolic activation S9. The applicant has chosen to given a long name (SET) equal to "ST+S9_C0". Set A1 is associated with the first row in the sample report table for study 123. |
|---|---|
| Rows 25-47: | Show trial set parameters and trial set values that comprise the test conditions for trial set A2. Set A2 is the data for the short term exposure with metabolic activation S9 at a concentration of 1250 ug/ml. The applicant has chosen to give the set a long name (SET) equal to "ST+S9_C1250". Set A2 is associated with the second row in the sample report table for study 123. |
tx.xpt
tx.xpt.xpt
| Row | STUDYID | GNTXAID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 123 | MNvit | TX | A1 | ST+S9_C0 | 2 | METACT | Metabolic Activation | +S9 |
| 2 | 123 | MNvit | TX | A1 | ST+S9_C0 | 3 | METACTFL | Y/N presence of metabolic activation | Y |
| 3 | 123 | MNvit | TX | A1 | ST+S9_C0 | 4 | TRTDMIN | Treatment Duration Minimum | 3 |
| 4 | 123 | MNvit | TX | A1 | ST+S9_C0 | 5 | TRTDTRG | Treatment Duration Target | 3.5 |
| 5 | 123 | MNvit | TX | A1 | ST+S9_C0 | 6 | TRTDMAX | Treatment Duration Maximum | 4 |
| 6 | 123 | MNvit | TX | A1 | ST+S9_C0 | 7 | TRTDU | Treatment Duration Unit | HOURS |
| 7 | 123 | MNvit | TX | A1 | ST+S9_C0 | 8 | RCVDMIN | Recovery Duration Minimum | 23.5 |
| 8 | 123 | MNvit | TX | A1 | ST+S9_C0 | 9 | RCVDTRG | Recovery Duration Target | 24 |
| 9 | 123 | MNvit | TX | A1 | ST+S9_C0 | 10 | RCVDMAX | Recovery Duration Maximum | 24.5 |
| 10 | 123 | MNvit | TX | A1 | ST+S9_C0 | 11 | RCVDU | Recovery Duration Unit | HOURS |
| 11 | 123 | MNvit | TX | A1 | ST+S9_C0 | 12 | INCBTMP | Incubation Temperature | 37 |
| 12 | 123 | MNvit | TX | A1 | ST+S9_C0 | 13 | INCBTMPU | Incubation Temperature Unit | C |
| 13 | 123 | MNvit | TX | A1 | ST+S9_C0 | 14 | HUMID | Atmospheric Relative Humidity Percent | 50 |
| 14 | 123 | MNvit | TX | A1 | ST+S9_C0 | 15 | ATMCO2 | Atmospheric CO2 Percent | 5 |
| 15 | 123 | MNvit | TX | A1 | ST+S9_C0 | 16 | SPTOBID | Sponsor defined tobacco identifier | CIG01a |
| 16 | 123 | MNvit | TX | A1 | ST+S9_C0 | 17 | EXPTYP | Exposure Type | Submerged |
| 17 | 123 | MNvit | TX | A1 | ST+S9_C0 | 18 | SAMTYP | Sample Type | Total Particulate Matter in DMSO |
| 18 | 123 | MNvit | TX | A1 | ST+S9_C0 | 19 | INTRVN | Name of the Intervention Article | Tobacco ProdA |
| 19 | 123 | MNvit | TX | A1 | ST+S9_C0 | 20 | ITVTYPE | type of intervention article | Negative Control |
| 20 | 123 | MNvit | TX | A1 | ST+S9_C0 | 21 | ITVCONC | Concentration of intervention article | 0 |
| 21 | 123 | MNvit | TX | A1 | ST+S9_C0 | 22 | ITVCONCU | Concentration Unit | ug/ml |
| 22 | 123 | MNvit | TX | A1 | ST+S9_C0 | 23 | SPDEVID | Sponsor defined device identifier | PUFFMASTER3K |
| 23 | 123 | MNvit | TX | A1 | ST+S9_C0 | 24 | DUREFID | Smoke Regimen | Medium Intensity Regimen |
| 24 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 2 | METACT | Metabolic Activation | +S9 |
| 25 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 3 | METACTFL | Y/N presence of metabolic activation | Y |
| 26 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 4 | TRTDMIN | Treatment Duration Minimum | 3 |
| 27 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 5 | TRTDTRG | Treatment Duration Target | 3.5 |
| 28 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 6 | TRTDMAX | Treatment Duration Maximum | 4 |
| 29 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 7 | TRTDU | Treatment Duration Unit | HOURS |
| 30 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 8 | RCVDMIN | Recovery Duration Minimum | 23.5 |
| 31 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 9 | RCVDTRG | Recovery Duration Target | 24 |
| 32 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 10 | RCVDMAX | Recovery Duration Maximum | 24.5 |
| 33 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 11 | RCVDU | Recovery Duration Unit | HOURS |
| 34 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 12 | INCBTMP | Incubation Temperature | 37 |
| 35 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 13 | INCBTMPU | Incubation Temperature Unit | C |
| 36 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 14 | HUMID | Atmospheric Relative Humidity Percent | 50 |
| 37 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 15 | ATMCO2 | Atmospheric CO2 Percent | 5 |
| 38 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 16 | SPTOBID | Sponsor defined tobacco identifier | CIG01a |
| 39 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 17 | EXPTYP | Exposure Type (See TIG NC workstream minutes 30-Jan here: Nonclinical) | Submerged |
| 40 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 18 | SAMTYP | Sample Type (e.g. TPM, GVP, whole aerosol, whole smoke conditioned media, aqueous extracts, etc., see notes here: Nonclinical) | Total Particulate Matter in DMSO |
| 41 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 19 | INTRVN | Name of the Intervention Article | Tobacco ProdA |
| 42 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 20 | ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Product |
| 43 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 21 | ITVCONC | Concentration of intervention article | 1250 |
| 44 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 22 | ITVCONCU | Concentration Unit | ug/ml |
| 45 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 23 | SPDEVID | Sponsor defined device identifier | PUFFMASTER2023 |
| 46 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 24 | DUREFID | Smoke Regimen | High Intensity Regimen |
Dataset Debug Messages
- There are 11 leading, trailing, or non-breaking spaces in the dataset.
- Please remove all hyperlinks from the dataset.
- Please remove all paragraph and/or line breaks.
Dataset Wrapper Debug Message
Please add a row column to your dataset.
A1: 
A2: 
| Row | STUDYID | ASSAYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|---|---|---|---|---|---|---|---|---|
| ST+S9_C0 | ||||||||
| 2 | 123 | MNvit | TX | A1 | ST+S9_C0 | 2 | METACT | Metabolic Activation (this is the type of activation used) | +S9 |
| 3 | 123 | MNvit | TX | A1 | ST+S9_C0 | 3 | METACTFL | Y/N presence of metabolic activation (this indicates that metabolic activation was used) | Y |
| 4 | 123 | MNvit | TX | A1 | ST+S9_C0 | 4 | TRTDMIN | Treatment Duration Minimum | 3 |
| 5 | 123 | MNvit | TX | A1 | ST+S9_C0 | 5 | TRTDTRG | Treatment Duration Target | 3.5 |
| 6 | 123 | MNvit | TX | A1 | ST+S9_C0 | 6 | TRTDMAX | Treatment Duration Maximum | 4 |
| 7 | 123 | MNvit | TX | A1 | ST+S9_C0 | 7 | TRTDU | Treatment Duration Unit | HOURS |
| 8 | 123 | MNvit | TX | A1 | ST+S9_C0 | 8 | RCVDMIN | Recovery Duration Minimum | 23.5 |
| 9 | 123 | MNvit | TX | A1 | ST+S9_C0 | 9 | RCVDTRG | Recovery Duration Target | 24 |
| 10 | 123 | MNvit | TX | A1 | ST+S9_C0 | 10 | RCVDMAX | Recovery Duration Maximum | 24.5 |
| 11 | 123 | MNvit | TX | A1 | ST+S9_C0 | 11 | RCVDU | Recovery Duration Unit | HOURS |
| 12 | 123 | MNvit | TX | A1 | ST+S9_C0 | 12 | INCBTMP | Incubation Temperature | 37 |
| 13 | 123 | MNvit | TX | A1 | ST+S9_C0 | 13 | INCBTMPU | Incubation Temperature Unit | C |
| 14 | 123 | MNvit | TX | A1 | ST+S9_C0 | 14 | HUMID | Atmospheric Relative Humidity Percent | 50 |
| 15 | 123 | MNvit | TX | A1 | ST+S9_C0 | 15 | ATMCO2 | Atmospheric CO2 Percent | 5 |
| 16 | 123 | MNvit | TX | A1 | ST+S9_C0 | 16 | SPTOBID | Sponsor defined tobacco identifier | CIG01a |
| 17 | 123 | MNvit | TX | A1 | ST+S9_C0 | 17 | EXPTYP | Exposure Type | Submerged |
| 18 | 123 | MNvit | TX | A1 | ST+S9_C0 | 18 | SAMTYP | Sample Type | Total Particulate Matter in DMSO |
| 19 | 123 | MNvit | TX | A1 | ST+S9_C0 | 19 | INTRVN | Name of the Intervention Article | Tobacco ProdA |
| 20 | 123 | MNvit | TX | A1 | ST+S9_C0 | 20 | ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Negative Control |
| 21 | 123 | MNvit | TX | A1 | ST+S9_C0 | 21 | ITVCONC | Concentration of intervention article | 0 |
| 22 | 123 | MNvit | TX | A1 | ST+S9_C0 | 22 | ITVCONCU | Concentration Unit | ug/ml |
| 23 | 123 | MNvit | TX | A1 | ST+S9_C0 | 23 | SPDEVID | Sponsor defined device identifier | PUFFMASTER3K |
| 24 | 123 | MNvit | TX | A1 | ST+S9_C0 | 24 | DUREFID | Smoke Regimen | Medium Intensity Regimen |
| 25 | 123 | MNvit | TX | A2 (table 1, row 2, ST exposure with S9 at concentration 1250) | ST+S9_C1250 | 1 | TESTSYS | Test system | TK6 Lymphoblastoid Suspension Cells |
| 26 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 2 | METACT | Metabolic Activation (this is the type of activation used) | +S9 |
| 27 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 3 | METACTFL | Y/N presence of metabolic activation (this indicates that metabolic activation was used) | Y |
| 28 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 4 | TRTDMIN | Treatment Duration Minimum | 3 |
| 29 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 5 | TRTDTRG | Treatment Duration Target | 3.5 |
| 30 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 6 | TRTDMAX | Treatment Duration Maximum | 4 |
| 31 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 7 | TRTDU | Treatment Duration Unit | HOURS |
| 32 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 8 | RCVDMIN | Recovery Duration Minimum | 23.5 |
| 33 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 9 | RCVDTRG | Recovery Duration Target | 24 |
| 34 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 10 | RCVDMAX | Recovery Duration Maximum | 24.5 |
| 35 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 11 | RCVDU | Recovery Duration Unit | HOURS |
| 36 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 12 | INCBTMP | Incubation Temperature | 37 |
| 37 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 13 | INCBTMPU | Incubation Temperature Unit | C |
| 38 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 14 | HUMID | Atmospheric Relative Humidity Percent | 50 |
| 39 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 15 | ATMCO2 | Atmospheric CO2 Percent | 5 |
| 40 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 16 | SPTOBID | Sponsor defined tobacco identifier | CIG01a |
| 41 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 17 | EXPTYP | Exposure Type (See TIG NC workstream minutes 30-Jan here: Nonclinical) | Submerged |
| 42 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 18 | SAMTYP | Sample Type (e.g. TPM, GVP, whole aerosol, whole smoke conditioned media, aqueous extracts, etc., see notes here: Nonclinical) | Total Particulate Matter in DMSO |
| 43 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 19 | INTRVN | Name of the Intervention Article | Tobacco ProdA |
| 44 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 20 | ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Product |
| 45 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 21 | ITVCONC | Concentration of intervention article | 1250 |
| 46 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 22 | ITVCONCU | Concentration Unit | ug/ml |
| 47 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 23 | SPDEVID | Sponsor defined device identifier | PUFFMASTER2023 |
| 48 | 123 | MNvit | TX | A2 | ST+S9_C1250 | 24 | DUREFID | Smoke Regimen | High Intensity Regimen |
| Row | STUDYID | ASSAYID | DOMAIN | SETCD | EUID | OBUID |
|---|---|---|---|---|---|---|
| 1 | 123 | MNvit | EI | A1 | C0 | |
| 2 | 123 | MNvit | EI | A2 | C1250 |
A1: 
A2: 
- Different runs would be expected to have different ENID and GTDTC values. In ei.xpt, these are distinguished by different RUNID values.
| Row | STUDYID | GNTXAID | DOMAIN | EUID | OBUID | REFID | GTSEQ | GTTESTCD | GTTEST | GTCELLEV (cells evaluated) | GTORRES | GTORRESU | GTCOLSRT ??? | GTSTRESC | GTSTRESN | GTSTRESU | GTDTC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 123 | MNvit | GT | C0 | 1 | RICC | Relative Increase in Cell Count | 154 | 0 | % | 0 | 0 | % | 2022-05-25 | |||
| 2 | 123 | MNvit | GT | C0 | 2 | RCC | Relative Cell Count | 154 | 0 | % | 0 | 0 | % | 2022-05-25 | |||
| 3 | 123 | MNvit | GT | C0 | 3 | RPD | Relative Population Doubling | 154 | 0 | % | 0 | 0 | % | 2022-05-25 | |||
| 4 | 123 | MNvit | GT | C0 | 4 | MNCELLS | Micronucleated Cells | 2205 | 15 | Cells | 15 | 15 | Cells | 2022-05-25 | |||
| 5 | 123 | MNvit | GT | C0 | 5 | MNCELLS | Micronucleated Cells | 2474 | 13 | Cells | 13 | 13 | Cells | 2022-05-25 | |||
| 6 | 123 | MNvit | GT | C0 | 6 | MNCELLS | Micronucleated Cells | 2758 | 17 | Cells | 17 | 17 | Cells | 2022-05-25 | |||
| 7 | 123 | MNvit | GT | C0 | 7 | MNCELLS | Micronucleated Cells | 2669 | 12 | Cells | 12 | 12 | Cells | 2022-05-25 | |||
| 8 | 123 | MNvit | GT | C0 | 8 | AVGREL | Average Relative MN Frequency | 0.57 | % | 0.57 | 0.57 | % | 2022-05-25 | ||||
| 9 | 123 | MNvit | GT | C1250 | 1 | RICC | Relative Increase in Cell Count | 134 | 15.7 | % | 15.7 | 15.7 | % | 2022-05-25 | |||
| 10 | 123 | MNvit | GT | C1250 | 2 | RCC | Relative Cell Count | 134 | 13.0 | % | 13.0 | 13.0 | % | 2022-05-25 | |||
| 11 | 123 | MNvit | GT | C1250 | 3 | RPD | Relative Population Doubling | 134 | 7.9 | % | 7.9 | 7.9 | % | 2022-05-25 | |||
| 12 | 123 | MNvit | GT | C1250 | 4 | MNCELLS | Micronucleated Cells | 3266 | 20 | Cells | 20 | 20 | Cells | 2022-05-25 | |||
| 13 | 123 | MNvit | GT | C1250 | 5 | MNCELLS | Micronucleated Cells | 2190 | 17 | Cells | 17 | 17 | Cells | 2022-05-25 | |||
| 14 | 123 | MNvit | GT | C1250 | 6 | MNCELLS | Micronucleated Cells | 2758 | 13 | Cells | 13 | 13 | Cells | 2022-05-25 | |||
| 15 | 123 | MNvit | GT | C1250 | 7 | MNCELLS | Micronucleated Cells | 2714 | 21 | Cells | 21 | 21 | Cells | 2022-05-25 | |||
| 16 | 123 | MNvit | GT | C1250 | 8 | AVGREL | Average Relative MN Frequency | 0.66 | % | 0.66 | 0.66 | % | 2022-05-25 |
- No labels