- Created by Lou Ann Kramer, last modified on Mar 05, 2023
You are viewing an old version of this page. View the current version.
Compare with Current View Page History
« Previous Version 31 Next »
- How do we handle "toxic, no revertant colonies"? (LAK will add 6 records with GTSTAT = NOT DONE, GTREASND = "TOO MUCH CYTOTOXICITY,"
- When "-" in Mean, Std Dev, Fold Inc - refer to SEND team's marked-up table (GTSTAT = NOT DONE; variety of REASND)
- populate units of COLONIES
- recreate the data table - clean up, add "S" to the first set A
- move assumptions out of all of the examples
- add row captions to highlight exceptions/assumptions
This is an example showing trial design and results from the in vitro bacterial reverse mutation test of Study #8325064. The bacterial reverse mutation test uses four different amino acid-requiring strains of Salmonella typhimurium (S. typhimurium) and one strain of Escherichia coli (E. coli) to detect point mutations, which involve substitution, addition or deletion of one or a few DNA base pairs. The principle of this bacterial reverse mutation test is that it detects chemicals that induce mutations which revert mutations present in the S typhimurium and E. coli tester strains and restore the functional capability of the bacteria to synthesize an essential amino acid. The revertant bacteria are detected by their ability to grow in the absence of the amino acid required by the parent tester strain.

- Assumption: When the experimental unit is derived from the species and strain, such as a cell line, then TESTSYS should be supplied.
Row | STUDYID | ASSAYID | DOMAIN | TSSEQ | TSGRPID | TSPARMCD | TSPARM | TSVAL | TSVALNF |
|---|---|---|---|---|---|---|---|---|---|
1 | 8325064 | Ames | TS | 1 | GLPTYP | Good Laboratory Practice Type | FDA | ||
2 | 8325064 | Ames | TS | 2 | GLPTYP | Good Laboratory Practice Type | OECD | ||
3 | 8325064 | Ames | TS | 1 | STSTDTC | Study Start Date | 2015-07-29 | ||
4 | 8325064 | Ames | TS | 1 | STITLE | Study Title | The Bacterial Reverse Mutation Test, Study 8325064-1 | ||
5 | 8325064 | Ames | TS | 1 | SNDIGVER | SEND Implementation Guide Version | TOBACCO IMPLEMENTATION GUIDE VERSION 1.0 | ||
6 | 8325064 | Ames | TS | 1 | SNDCTVER | SEND Controlled Terminology Version | SEND Terminology 2021-09-30 | ||
7 | 8325064 | Ames | TS | 1 | SSPONSOR | Sponsor Organization | Example Sponsor Inc. | ||
8 | 8325064 | Ames | TS | 1 | SPREFID | Sponsor's Study Reference ID | NOT APPLICABLE | ||
9 | 8325064 | Ames | TS | 1 | 1 | TSTFNAM | Test Facility Name | Example Tox Lab Name | |
10 | 8325064 | Ames | TS | 1 | 1 | TSTFLOC | Test Facility Location | 10 Somewhere Street, Montgomery, AL 10000 | |
11 | 8325064 | Ames | TS | 1 | 1 | TFCNTRY | Test Facility Country | USA | |
12 | 8325064 | Ames | TS | 1 | 1 | STDIR | Study Director | Dr. R. Smith | |
13 | 8325064 | Ames | TS | 1 | GLPFL | GLP Flag | N | ||
14 | 8325064 | Ames | TS | 1 | ASTD | Assay Standard | OECD Test No. 471 | ||
15 | 8325064 | Ames | TS | 1 | ASTDV | Assay Standard Version | 2020-06-29 | ||
16 | 8325064 | Ames | TS | 1 | SSTYP | Study Type | GENOTOXICITY IN VITRO | ||
17 | 8325064 | Ames | TS | 1 | SSSTYP | Study Sub Type | Bacterial Reverse Mutation Test | ||
18 | 8325064 | Ames | TS | 1 | 2 | SPECIES | Species | Salmonella | |
19 | 8325064 | Ames | TS | 1 | 2 | STRAIN | Strain | TA98 | |
20 | 8325064 | Ames | TS | 1 | 2 | STRAIN | Strain | TA100 | |
21 | 8325064 | Ames | TS | 1 | 2 | STRAIN | Strain | TA1535 | |
22 | 8325064 | Ames | TS | 1 | 2 | STRAIN | Strain | TA1537 | |
23 | 8325064 | Ames | TS | 1 | 3 | SPECIES | Species | Escherichia coli | |
24 | 8325064 | Ames | TS | 1 | 3 | STRAIN | Strain | WP2 uvrA pKM101 |
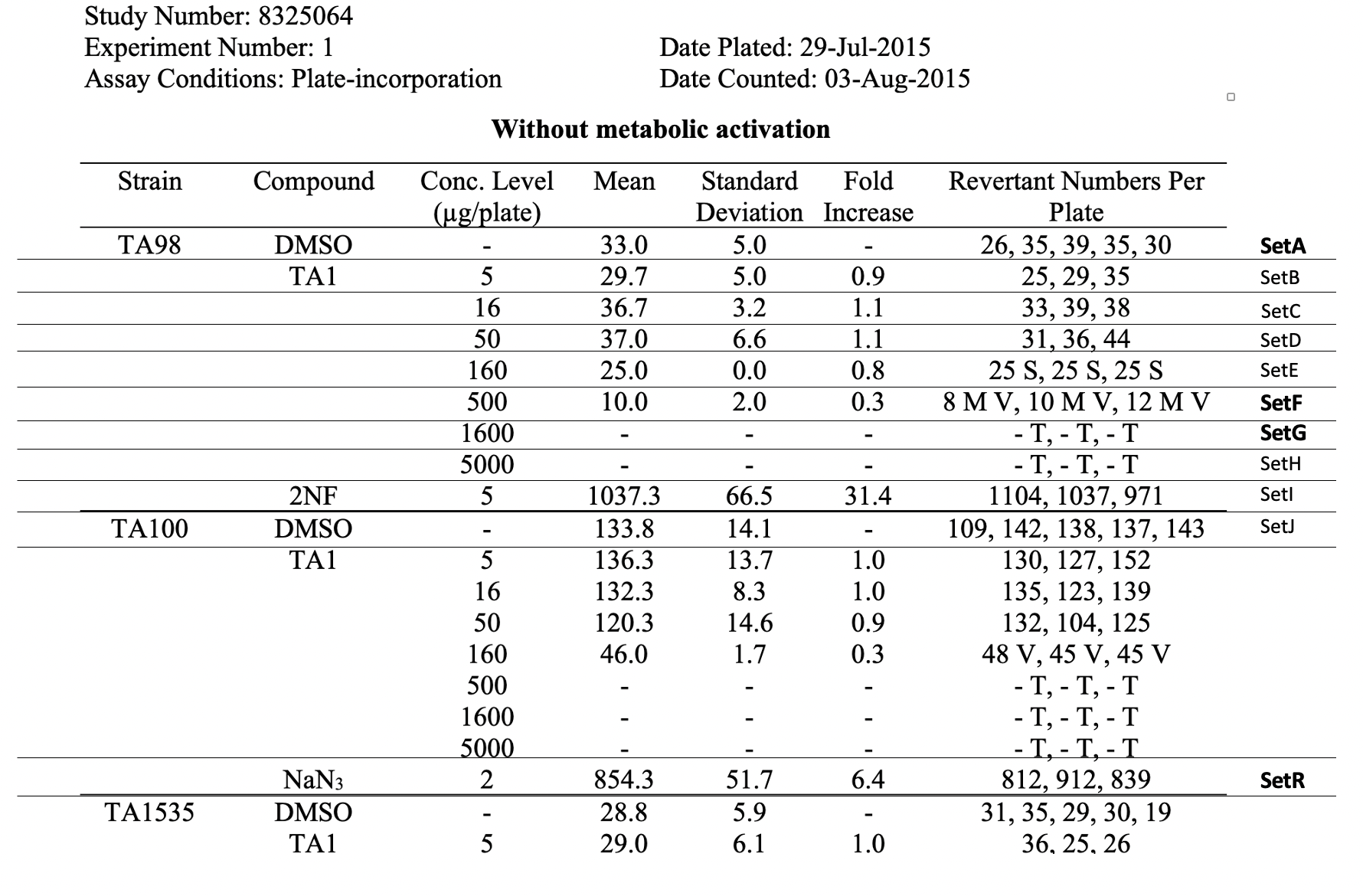
| Rows 1-22: | Show trial set parameters and trial set values that comprise the test conditions for the set SetA. SetA is the first row of the raw data table, see the row labeled "SetA" in the table, Raw Data for Study 8325064. |
|---|---|
| Rows 2-4: | Show two records for Alkaline Phosphatase that were collected 1 day apart and are expected to be reported together. Row 4 shows how to create a derived record (average of the Rows 2 and 3) and flag it as Derived (LBDRVFL = "Y") as well as the record to use as baseline (LBBLFL = "Y"). |
| Rows 6-7: | Show a suggested use of the LBSCAT variable. It could be used to further classify types of tests within a laboratory panel (e.g., "DIFFERENTIAL"). |
| Row 13: | Shows the use of LBUSCHFL to indicate that the test result was obtained from an unscheduled blood collection. In this case, the subject was moribund and a blood sample was taken prior to sacrifice. |
| Rows 1, 6: | Use records in the SUPPLB dataset example to show biological significance assigned by the investigator for test results. |
tx.xpt trial sets.xpt
| Row | STUDYID | ASSAYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 1 | SPECIES | Species | Salmonella typhimurium |
| 2 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 2 | STRAIN | Strain/Substrain | TA98 |
| 3 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 3 | METACT | Metabolic Activation | |
| 4 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 4 | METACTFL | Y/N presence of metabolic activation | N |
| 5 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 5 | TRTDMIN | Treatment Duration Minimum | 71.5 |
| 6 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 6 | TRTDTRG | Treatment Duration Target | 72 |
| 7 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 7 | TRTDMAX | Treatment Duration Maximum | 72.5 |
| 8 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 8 | TRTDU | Treatment Duration Unit | HOURS |
| 9 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 9 | INCBTMP | Incubation Temperature | 37 |
| 10 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 10 | INCBTMPU | Incubation Temperature Unit | C |
| 11 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 11 | HUMID | Atmospheric Relative Humidity Percent | 50 |
| 12 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 12 | ATMCO2 | Atmospheric CO2 Percent | 5 |
| 13 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 13 | SPTOBID | Sponsor defined tobacco identifier | CIG01a |
| 14 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 14 | EXPTYP | Exposure Type (See TIG NC workstream minutes 30-Jan here: Nonclinical) | Submerged |
| 15 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 15 | SAMTYP | Sample Type | Total Particulate Matter in PBS |
| 16 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 16 | INTRVN | name of the intervention article | TA1 |
| 17 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 17 | ITVTYPE | type of intervention article | Vehicle Control |
| 18 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 18 | ITVCONC | Concentration of intervention article | 0 |
| 19 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 19 | ITVCONCU | Concentration Unit | ug/plate |
| 20 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 20 | TRTV | Treatment Vehicle | DMSO |
| 21 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 21 | SPDEVID | Sponsor defined device identifier | PUFFMASTER3K |
| 22 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 22 | DUREFID | Smoke Regimen | Medium Intensity Regimen |
| 23 | 8325064 | Ames | TX | SetF | F-TA98-C500 | 1 | SPECIES | Species | |
| 24 | 8325064 | Ames | TX | SetF | F-TA98-C500 | 2 | STRAIN | Strain/Substrain | |
| 25 | 8325064 | Ames | TX | SetF | F-TA98-C500 | 3 | METACT | Metabolic Activation | |
| 26 | 8325064 | Ames | TX | SetF | F-TA98-C500 | 4 | METACTFL | Y/N presence of metabolic activation | N |
| 27 | 8325064 | Ames | TX | SetF | F-TA98-C500 | TRTDRTOL | Treatment Duration Tolerance | ||
| 28 | 8325064 | Ames | TX | SetF | F-TA98-C500 | TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | ||
| 8325064 | Ames | TX | SetF | F-TA98-C500 | INTRVN | name of the intervention article (Tobacco ProdA, Bleomycin or Cyclophosphamid A) | TA1 | ||
| 8325064 | Ames | TX | SetF | F-TA98-C500 | ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Product | ||
| 8325064 | Ames | TX | SetF | F-TA98-C500 | ITVCONC | Concentration of intervention article | 500 | ||
| 8325064 | Ames | TX | SetF | F-TA98-C500 | ITVCONCU | Concentration Unit | ug/plate | ||
| 8325064 | Ames | TX | SetF | F-TA98-C500 | STRAIN | Strain/Substrain | TA98 | ||
| 8325064 | Ames | TX | SetF | F-TA98-C500 | REGIME | Smoking Regime | ISO Regime | ||
| ... | |||||||||
| 8325064 | Ames | TX | SetG (row 7) do G not I!!! | METACT | Metabolic Activation (should there be two parms? Presence, type)? | None | |||
| 8325064 | Ames | TX | SetG | TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) | ||||
| 8325064 | Ames | TX | SetG | TRTDRTOL | Treatment Duration Tolerance | ||||
| 8325064 | Ames | TX | SetG | TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | ||||
| 8325064 | Ames | TX | SetG | INTRVN | name of the intervention article (Tobacco ProdA, Bleomycin or Cyclophosphamid A) | 2-Nitrofluorine | |||
| 8325064 | Ames | TX | SetG | ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Positive Control | |||
| 8325064 | Ames | TX | SetG | ITVCONC | Concentration of intervention article | 5 | |||
| 8325064 | Ames | TX | SetG | ITVCONCU | Concentration Unit | ug/plate | |||
| 8325064 | Ames | TX | SetG | REGIME | Smoking Regime | ISO | |||
| ... | SetG | ||||||||
| 8325064 | Ames | TX | SetR (Row 18) | METACT | Metabolic Activation (should there be two parms? Presence, type)? | None | |||
| 8325064 | Ames | TX | SetR | TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) | ||||
| 8325064 | Ames | TX | SetR | TRTDRTOL | Treatment Duration Tolerance | ||||
| 8325064 | Ames | TX | SetR | TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | ||||
| 8325064 | Ames | TX | SetR | INTRVN | name of the intervention article (Tobacco ProdA, Bleomycin or Cyclophosphamid A) | 4-Nitroquinoline-1-oxide | |||
| 8325064 | Ames | TX | SetR | ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Positive Control | |||
| 8325064 | Ames | TX | SetR | ITVCONC | Concentration of intervention article | 2 | |||
| 8325064 | Ames | TX | SetR | ITVCONCU | Concentration Unit | ug/plate | |||
| 8325064 | Ames | TX | SetR | STRAIN | Strain/Substrain | TA98 | |||
| 8325064 | Ames | TX | SetR | REGIME | Smoking Regime | ISO |
Dataset Wrapper Debug Message
Please add a row column to your dataset.
| Row | STUDYID | ASSAYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 1 | SPECIES | Species | Salmonella typhimurium |
| 2 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 2 | STRAIN | Strain/Substrain | TA98 |
| 3 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 3 | METACT | Metabolic Activation | |
| 4 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 4 | METACTFL | Y/N presence of metabolic activation | N |
| 5 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 5 | TRTDMIN | Treatment Duration Minimum | 71.5 |
| 6 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 6 | TRTDTRG | Treatment Duration Target | 72 |
| 7 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 7 | TRTDMAX | Treatment Duration Maximum | 72.5 |
| 8 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 8 | TRTDU | Treatment Duration Unit | HOURS |
| 9 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 9 | INCBTMP | Incubation Temperature | 37 |
| 10 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 10 | INCBTMPU | Incubation Temperature Unit | C |
| 11 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 11 | HUMID | Atmospheric Relative Humidity Percent | 50 |
| 12 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 12 | ATMCO2 | Atmospheric CO2 Percent | 5 |
| 13 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 13 | SPTOBID | Sponsor defined tobacco identifier | CIG01a |
| 14 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 14 | EXPTYP | Exposure Type (See TIG NC workstream minutes 30-Jan here: Nonclinical) | Submerged |
| 15 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 15 | SAMTYP | Sample Type | Total Particulate Matter in PBS |
| 16 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 16 | INTRVN | name of the intervention article | TA1 |
| 17 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 17 | ITVTYPE | type of intervention article | Vehicle Control |
| 18 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 18 | ITVCONC | Concentration of intervention article | 0 |
| 19 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 19 | ITVCONCU | Concentration Unit | ug/plate |
| 20 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 20 | TRTV | Treatment Vehicle | DMSO |
| 21 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 21 | SPDEVID | Sponsor defined device identifier | PUFFMASTER3K |
| 22 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 22 | DUREFID | Smoke Regimen | Medium Intensity Regimen |
| 23 | 8325064 | Ames | TX | SetF | F-TA98-C500 | 1 | SPECIES | Species | |
| 24 | 8325064 | Ames | TX | SetF | F-TA98-C500 | 2 | STRAIN | Strain/Substrain | |
| 25 | 8325064 | Ames | TX | SetF | F-TA98-C500 | 3 | METACT | Metabolic Activation | |
| 26 | 8325064 | Ames | TX | SetF | F-TA98-C500 | 4 | METACTFL | Y/N presence of metabolic activation | N |
| 27 | 8325064 | Ames | TX | SetF | F-TA98-C500 | TRTDRTOL | Treatment Duration Tolerance | ||
| 28 | 8325064 | Ames | TX | SetF | F-TA98-C500 | TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | ||
| 8325064 | Ames | TX | SetF | F-TA98-C500 | INTRVN | name of the intervention article (Tobacco ProdA, Bleomycin or Cyclophosphamid A) | TA1 | ||
| 8325064 | Ames | TX | SetF | F-TA98-C500 | ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Product | ||
| 8325064 | Ames | TX | SetF | F-TA98-C500 | ITVCONC | Concentration of intervention article | 500 | ||
| 8325064 | Ames | TX | SetF | F-TA98-C500 | ITVCONCU | Concentration Unit | ug/plate | ||
| 8325064 | Ames | TX | SetF | F-TA98-C500 | STRAIN | Strain/Substrain | TA98 | ||
| 8325064 | Ames | TX | SetF | F-TA98-C500 | REGIME | Smoking Regime | ISO Regime | ||
| ... | |||||||||
| 8325064 | Ames | TX | SetG (row 7) do G not I!!! | METACT | Metabolic Activation (should there be two parms? Presence, type)? | None | |||
| 8325064 | Ames | TX | SetG | TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) | ||||
| 8325064 | Ames | TX | SetG | TRTDRTOL | Treatment Duration Tolerance | ||||
| 8325064 | Ames | TX | SetG | TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | ||||
| 8325064 | Ames | TX | SetG | INTRVN | name of the intervention article (Tobacco ProdA, Bleomycin or Cyclophosphamid A) | 2-Nitrofluorine | |||
| 8325064 | Ames | TX | SetG | ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Positive Control | |||
| 8325064 | Ames | TX | SetG | ITVCONC | Concentration of intervention article | 5 | |||
| 8325064 | Ames | TX | SetG | ITVCONCU | Concentration Unit | ug/plate | |||
| 8325064 | Ames | TX | SetG | REGIME | Smoking Regime | ISO | |||
| ... | SetG | ||||||||
| 8325064 | Ames | TX | SetR (Row 18) | METACT | Metabolic Activation (should there be two parms? Presence, type)? | None | |||
| 8325064 | Ames | TX | SetR | TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) | ||||
| 8325064 | Ames | TX | SetR | TRTDRTOL | Treatment Duration Tolerance | ||||
| 8325064 | Ames | TX | SetR | TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | ||||
| 8325064 | Ames | TX | SetR | INTRVN | name of the intervention article (Tobacco ProdA, Bleomycin or Cyclophosphamid A) | 4-Nitroquinoline-1-oxide | |||
| 8325064 | Ames | TX | SetR | ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Positive Control | |||
| 8325064 | Ames | TX | SetR | ITVCONC | Concentration of intervention article | 2 | |||
| 8325064 | Ames | TX | SetR | ITVCONCU | Concentration Unit | ug/plate | |||
| 8325064 | Ames | TX | SetR | STRAIN | Strain/Substrain | TA98 | |||
| 8325064 | Ames | TX | SetR | REGIME | Smoking Regime | ISO |
Unable to render row captions
Please move all content other than the row captions outside of the macro.
NOTE: Observational Unit Identifiers (OBUIDs) are defined by the sponsor to uniquely identify the observational unit within an experimental unit. In this example OBUIDs are defined by the sponsor based on the test conditions (trial set) and the unit's location on one of multiple exposure/incubation plates.
| Rows 1-5: | Show the values for OBUID within the experimental unit identifier for Set A, as defined in the trial set dataset. |
| Rows 6-8: | Show the values for OBUID within the experimental unit identifier for Set F, as defined in the trial set dataset. |
| Rows 9-11: | Show the values for OBUID within the experimental unit identifier for Set G, as defined in the trial set dataset. |
| Row 12-14: | Show the values for OBUID within the experimental unit identifier for Set R, as defined in the trial set dataset. |
xx.xpt
| Row | STUDYID | ASSAYID | DOMAIN | SETCD | EUID | OBUID |
|---|---|---|---|---|---|---|
| 1 | 8325064 | Ames | OU | SetA | A | 0_1 |
| 2 | 8325064 | Ames | OU | SetA | A | 0_2 |
| 3 | 8325064 | Ames | OU | SetA | A | 0_3 |
| 4 | 8325064 | Ames | OU | SetA | A | 0_4 |
| 5 | 8325064 | Ames | OU | SetA | A | 0_5 |
6 | 8325064 | Ames | OU | SetF | F | 6_1 |
| 7 | 8325064 | Ames | OU | SetF | F | 6_2 |
| 8 | 8325064 | Ames | OU | SetF | F | 6_3 |
| 9 | 8325064 | Ames | OU | SetG | G | 7_1 |
| 10 | 8325064 | Ames | OU | SetG | G | 7_2 |
| 11 | 8325064 | Ames | OU | SetG | G | 7_3 |
| 12 | 8325064 | Ames | OU | SetR | R | 18_1 |
| 13 | 8325064 | Ames | OU | SetR | R | 18_2 |
| 14 | 8325064 | Ames | OU | SetR | R | 18_3 |
| Rows 1-7: | Show values collected for the set, SetA. |
|---|---|
| Rows 8-15: | Shows values collected for the set, SetF. |
| Rows 6-7: | Show a suggested use of the LBSCAT variable. It could be used to further classify types of tests within a laboratory panel (e.g., "DIFFERENTIAL"). |
| Rows 6,7,12,13, x,x,x,x: | Show values collected (e.g., MEAN, STANDARD DEVIATION, and/or FOLD INCREASE) that apply to all of the observational units within the experimental unit, as show by OBUID = "ALL". |
| Row 9: | Shows the proper use of the LBSTAT variable to indicate "NOT DONE," when the fact that the test was done was documented. |
| Row 12: | The subject had Cholesterol measured. The normal range for this test is <200 mg/dL. The value <200 may not be used in the normal range variables LBORNRHI or LBSTNRHI; however, a sponsor may decide, e.g., to enter "0" into LBORNRLO and "199" in LBORNRHI. The sponsor must define the appropriate value for all of the normal range variables. |
| Row 13: | Shows the use of LBUSCHFL to indicate that the test result was obtained from an unscheduled blood collection. In this case, the subject was moribund and a blood sample was taken prior to sacrifice. |
| Rows 1, 6: | Use records in the SUPPLB dataset example to show biological significance assigned by the investigator for test results. |
Row Captions Debug Messages
- Remember to keep number agreement between labels and captions.
- Please construct row caption labels as instructed in <ac:link><ri:page ri:content-title="Constructing labels for row captions" ri:space-key="TTD"/></ac:link>.

gt.xpt.xpt
| Row | STUDYID | ASSAYID | DOMAIN | TXSETCD | EUID | OUID | GTSEQ | GTTESTCD | GTTEST | GTORRES | GTORRESU | GTCOLSRT (coll. summ. result type) | GTSTRESC | GTSTRESN | GTSTRESU | GTDTC | GTMETHOD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 8325064 | Ames | GT | SETA | A | 0_1 | 1 | RPP | Revertant Numbers Per Plate | 26 | COLONIES | 26 | 26 | COLONIES | 2015-08-03 | INSTRUMENT COUNTED | |
| 2 | 8325064 | Ames | GT | SETA | A | 0_2 | 2 | RPP | Revertant Numbers Per Plate | 35 | COLONIES | 35 | 35 | 2015-08-03 | INSTRUMENT COUNTED | ||
| 3 | 8325064 | Ames | GT | SETA | A | 0_3 | 3 | RPP | Revertant Numbers Per Plate | 39 | COLONIES | 39 | 39 | 2015-08-03 | INSTRUMENT COUNTED | ||
| 4 | 8325064 | Ames | GT | SETA | A | 0_4 | 4 | RPP | Revertant Numbers Per Plate | 35 | COLONIES | 35 | 35 | 2015-08-03 | INSTRUMENT COUNTED | ||
| 5 | 8325064 | Ames | GT | SETA | A | 0_5 | 5 | RPP | Revertant Numbers Per Plate | 30 | COLONIES | 30 | 30 | 2015-08-03 | INSTRUMENT COUNTED | ||
| 6 | 8325064 | Ames | GT | SETA | A | ALL | 6 | RPP | Revertant Numbers Per Plate | 33.0 | COLONIES | MEAN | 33.0 | 33.0 | |||
| 7 | 8325064 | Ames | GT | SETA | A | ALL | 7 | RPP | Revertant Numbers Per Plate | 5.0 | COLONIES | STANDARD DEVIATION | 5.0 | 5.0 | |||
| 8 | 8325064 | Ames | GT | SETF | F | 6_1 | 1 | RPP | Revertant Numbers Per Plate | 8 | COLONIES | 8 | 8 | MANUALLY COUNTED | |||
| 9 | 8325064 | Ames | GT | SETF | F | 6_1 | 2 | CYTOTOX | Cytotoxicity | Very thin background bacterial lawn | COLONIES | V | |||||
| 10 | 8325064 | Ames | GT | SETF | F | 6_2 | 3 | RPP | Revertant Numbers Per Plate | 10 | COLONIES | 10 | 10 | MANUALLY COUNTED | |||
| 11 | 8325064 | Ames | GT | SETF | F | 6_3 | 4 | RPP | Revertant Numbers Per Plate | 12 | COLONIES | 12 | 12 | MANUALLY COUNTED | |||
| 12 | 8325064 | Ames | GT | SETF | F | ALL | 5 | RPPMEAN | Mean of Revertant Numbers Per Plate | 10.0 | COLONIES | MEAN | 10.0 | 10.0 | |||
| 13 | 8325064 | Ames | GT | SETF | F | ALL | 6 | RPPSTDDV | Standard Deviation of Revertant Numbers Per Plate | 2.0 | COLONIES | STANDARD DEVIATION | 2.0 | 2.0 | |||
| 14 | 8325064 | Ames | GT | SETF | F | ALL | 7 | RPPFDINC | Fold Increase of Revertant Numbers Per Plate | 0.3 | COLONIES | FOLD INCREASE | 0.3 | 0.3 | |||
| 8325064 | Ames | GT | SETG | G | 5_1 | 1 | RPP | Revertant Numbers Per Plate | 1104 | 1104 | 1104 | ||||||
| 8325064 | Ames | GT | SETG | G | 5_2 | 2 | RPP | Revertant Numbers Per Plate | 1037 | 1037 | 1037 | ||||||
| 8325064 | Ames | GT | SETG | G | 5_3 | 3 | RPP | Revertant Numbers Per Plate | 971 | 971 | 971 | ||||||
| 8325064 | Ames | GT | SETR | R | ALL | RPPMEAN | Mean of Revertant Numbers Per Plate | 1037.3 | 1037.3 | 1037.3 | |||||||
| 8325064 | Ames | GT | SETR | R | ALL | RPPSTDDV | Standard Deviation of Revertant Numbers Per Plate | 66.5 | 66.5 | 66.5 | |||||||
| 8325064 | Ames | GT | SETR | R | ALL | RPPFLDINC | Fold Increase of Revertant Numbers Per Plate | 31.4 | 31.4 | 31.4 |
Dataset Wrapper Debug Message
Please add a row column to your dataset.
- No labels