- Created by Lou Ann Kramer, last modified on Jan 16, 2023
You are viewing an old version of this page. View the current version.
Compare with Current View Page History
« Previous Version 59 Next »
This is an example showing trial design and results data of Study #123 for the determination of the in vitro genotoxicity potential of 10 tobacco products in the in vitro Micronucleus Assay
- red font - indicates potential for CT code lists
- green font - links to other domains
- purple font - to be discussed
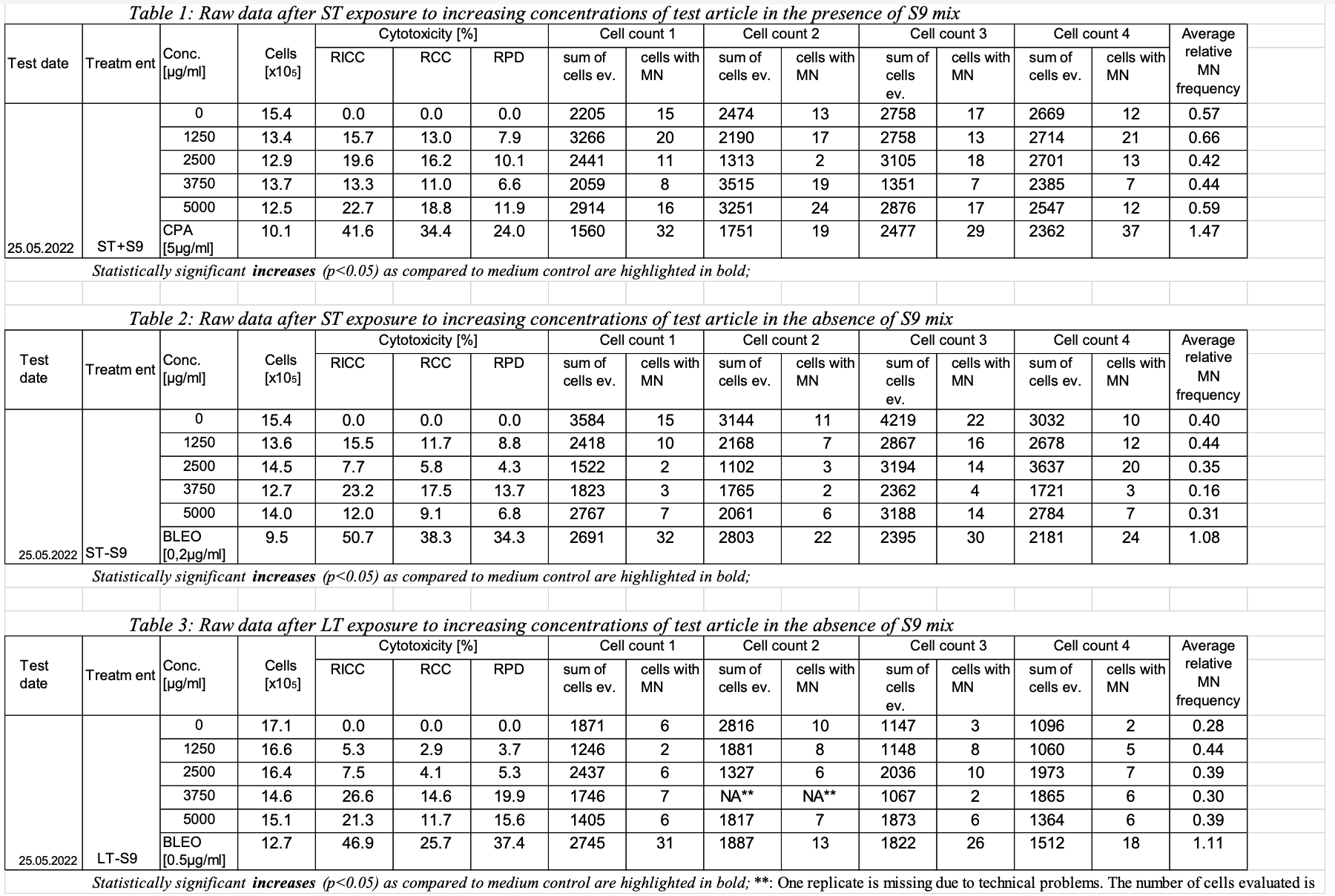
- Assumption: The intent of this dataset is to provide a summary of trial (study) information. This is not subject-level data.
- Assumption: A Trial (study) can have more than one assay type
- Assumption: ASSAYID value of ALL indicates that it applies to all assays in the study
- This example currently shows SPDEVID and DUREFID in BOTH the TS domain and the TX domain, but we should discuss if this should only ever be in TX (with the same value for all sets if there is only one device used)
- Assumption: SPDEVID (sponsor defined device identifier) should be added at the trial set level (tx.xpt) - we can discuss if this should be in TS when there is only one device for a study
- This allows for studies where there are multiple products and different product(s) per trial set, one record for each product that is being tested in each particular trial set (tx.xpt)
Row | STUDYID | ASSAYID | DOMAIN | TSSEQ | TSGRPID | TSPARMCD | TSPARM | TSVAL | TSVALNF |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 123 | MNvit | TS | 1 | GLPTYP | Good Laboratory Practice Type | FDA | ||
| 2 | 123 | MNvit | TS | 2 | GLPTYP | Good Laboratory Practice Type | OECD | ||
| 3 | 123 | MNvit | TS | 1 | STSTDTC | Study Start Date | 2022-05-25 | ||
| 4 | 123 | MNvit | TS | 1 | STITLE | Study Title | Determination of the in vitro genotoxicity potential of 10 tobacco products in the in vitro Micronucleus Assay | ||
| 5 | 123 | MNvit | TS | 1 | SNDIGVER | SEND Implementation Guide Version | TOBACCO IMPLEMENTATION GUIDE VERSION 1.0 | ||
| 6 | 123 | MNvit | TS | 1 | SNDCTVER | SEND Controlled Terminology Version | SEND Terminology 2021-09-30 | ||
| 7 | 123 | MNvit | TS | 1 | SSPONSOR | Sponsor Organization | Example Sponsor Inc. | ||
| 8 | 123 | MNvit | TS | 1 | SPREFID | Sponsor's Study Reference ID | NOT APPLICABLE | ||
| 9 | 123 | MNvit | TS | 1 | 1 | TSTFNAM | Test Facility Name | Example Tox Lab Name | |
| 10 | 123 | MNvit | TS | 1 | 1 | TSTFLOC | Test Facility Location | 10 Somewhere Street, Montgomery, AL 10000 | |
| 11 | 123 | MNvit | TS | 1 | 1 | TFCNTRY | Test Facility Country | USA | |
| 12 | 123 | MNvit | TS | 1 | 1 | STDIR | Study Director | Dr. R. Smith | |
| 13 | 123 | MNvit | TS | 1 | GLPFL | GLP Flag | Y | ||
| 14 | 123 | MNvit | TS | 1 | ASTD | Assay Standard | OECD Test No. 487 | ||
| 15 | 123 | MNvit | TS | 1 | ASTDV | Assay Standard Version | 2016-07-29 | ||
| 16 | 123 | MNvit | TS | 1 | SSTYP | Study Type | GENOTOXICITY IN VITRO | ||
| 17 | 123 | MNvit | TS | 1 | SSSTYP | Study Sub Type | In Vitro Micronucleus | ||
| 18 | 123 | MNvit | TS | 1 | SPECIES | Species | Homo Sapiens | ||
| 19 | 123 | MNvit | TS | 1 | ?? | Test System? | TK6 Lymphoblastoid Suspension Cells | ||
| 20 | 123 | MNvit | TS | 1 | SPDEVID | Sponsor defined device identifier | PUFFMASTER3K | ||
| 21 | 123 | MNvit | TS | 1 | DUREFID | Smoke Regimen | Medium Intensity Regimen |
- During CT definition/reviews will decide appropriate TXPARM and TXVAL; Treatment duration may be controlled; For now, we just include good example values based on our experience
- Assumption: The Trial Sets (TX) domain provides the list of distinct sets of subjects having different experimental factors, treatment factors, inherent characteristics, or distinct sponsor designations as specified in the trial design.
- Where is TK6 cell type? is this test system (see below)
- needs to be allowed to vary down to the well level / result level
A1: 
A2: 
| Row | STUDYID | ASSAYID | DOMAIN | SETCD | SET (what sponsor calls it) | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|---|---|---|---|---|---|---|---|---|
| 123 | MNvit | TX | A1 (table 1, row 1, ST exposure with S9) | METACT | Metabolic Activation (should there be two parms? Presence, type)? | +S9 | |||
| METACTFL | Y/N presence of metabolic activation | ||||||||
| 123 | MNvit | TX | A1 | TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) | 3 | |||
| 123 | MNvit | TX | A1 | TRTDRTOL | Treatment Duration Tolerance | ||||
| 123 | MNvit | TX | A1 | TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | H | |||
| 123 | MNvit | TX | A1 | INTRVN | name of the intervention article (Tobacco ProdA, Bleomycin or Cyclophosphamid A) | Tobacco ProdA | |||
| 123 | MNvit | TX | A1 | ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Product | |||
| 123 | MNvit | TX | A1 | ITVCONC | Concentration of intervention article | 0 | |||
| 123 | MNvit | TX | A1 | ITVCONCU | Concentration Unit | ug/ml | |||
| 123 | MNvit | TS | A1 | SPDEVID | Sponsor defined device identifier | PUFFMASTER3K | |||
| 123 | MNvit | TS | A1 | DUREFID | Smoke Regimen | Medium Intensity Regimen | |||
| 123 | MNvit | TX | A2 (table 1, row 2) | METACT | Metabolic Activation (should there be two parms? Presence, type)? | +S9 | |||
| 123 | MNvit | TX | A2 | TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) | 3 | |||
| 123 | MNvit | TX | A2 | TRTDRTOL | Treatment Duration Tolerance | ||||
| 123 | MNvit | TX | A2 | TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | H | |||
| 123 | MNvit | TX | A2 | INTRVN | name of the intervention article | Tobacco ProdA | |||
| 123 | MNvit | TX | A2 | ITVTYPE | type of intervention article | Product | |||
| 123 | MNvit | TX | A2 | ITVCONC | Concentration of i a | 1250 | |||
| 123 | MNvit | TX | A2 | ITVCONCU | Concentration Unit | ug/ml | |||
| 123 | MNvit | TS | A2 | SPDEVID | Sponsor defined device identifier | PUFFMASTER2023 | |||
| 123 | MNvit | TS | A2 | DUREFID | Smoke Regimen | High Intensity Regimen | |||
| ... | |||||||||
We use DU for smoking regimen. Note that a separate DI dataset will be needed to show identifying parameters of the "PUFFMASTER3K" smoking machine, but is currently not shown for brevity.
- Details of the smoking regimen are represented as device in-use properties, linked to the stability data in PT above by matching values of PTREFID/DUREFID = "Medium Intensity Regimen" (We will update with a realistic value for the regimen, with input).
- Smoking regimen is represented in --REFID (we made up a value of "Medium Intensity Regimen"; we can update with something realistic)
- The smoking regimen is carried out by the smoking machine/device shown in SPDEVID, Sponsor defined device identifier, "PUFFMASTER3K"
- Do we need both SPDEVID AND DUREFID?
du.xpt
Row | STUDYID | DOMAIN | SPDEVID | DUSEQ | DUREFID | DUGRPID | DUTESTCD | DUTEST | DUORRES | DUORRESU | DUSTRESC | DUSTRESN | DUSTRESU |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 123 | DU | PUFFMASTER3K | 1 | Medium Intensity Regimen | PUFFPROF | Puff Profile | SQUARE | SQUARE | ||||
| 2 | 123 | DU | PUFFMASTER3K | 2 | Medium Intensity Regimen | PUFFDUR | Puff Duration | 1.25 | sec | 1.25 | 1.25 | sec | |
| 3 | 123 | DU | PUFFMASTER3K | 3 | Medium Intensity Regimen | PUFFINT | Puff Interval | 3 | PUFF/min | 3 | 3 | PUFF/min | |
| 4 | 123 | DU | PUFFMASTER3K | 4 | Medium Intensity Regimen | PUFFBLCK | Puff Block | 25 | % | 25 | 25 | % | |
| 5 | 123 | DU | PUFFMASTER3K | 5 | Medium Intensity Regimen | NUMPUFF | Total Number of Puffs | 200 | PUFF | 200 | 200 | PUFF | |
| 6 | 123 | DU | PUFFMASTER3K | 6 | Medium Intensity Regimen | PUFFVOL | Puff Volume | 10 | mL | 10 | 10 | mL | |
| 7 | 123 | DU | PUFFMASTER3K | 7 | Medium Intensity Regimen | PUFFRNG | Puff Range | 100-200 | 100-200 | ||||
| 8 | 123 | DU | PUFFMASTER3K | 8 | Medium Intensity Regimen | 1 | PUFFPAUS | Puff Pause | 60 | s | 60 | 60 | s |
| 9 | 123 | DU | PUFFMASTER3K | 9 | Medium Intensity Regimen | 1 | PUFFPINT | Puff Pause Interval | 10 | PUFF | 10 | 10 | PUFF |
| 10 | 123 | DU | PUFFMASTER2023 | 1 | Canadian Intense Regime | PUFFPROF | Puff Profile | SQUARE | SQUARE | ||||
| 11 | 123 | DU | PUFFMASTER2023 | 2 | Canadian Intense Regime | PUFFDUR | Puff Duration | 2.00 | sec | 2.00 | 2.00 | sec | |
| 12 | 123 | DU | PUFFMASTER2023 | 3 | Canadian Intense Regime | PUFFINT | Puff Interval | 4 | PUFF/min | 4 | 4 | PUFF/min | |
| 13 | 123 | DU | PUFFMASTER2023 | 4 | Canadian Intense Regime | PUFFBLCK | Puff Block | 0 | % | 0 | 0 | % | |
| 14 | 123 | DU | PUFFMASTER2023 | 5 | Canadian Intense Regime | NUMPUFF | Total Number of Puffs | 200 | PUFF | 200 | 200 | PUFF | |
| 15 | 123 | DU | PUFFMASTER2023 | 6 | Canadian Intense Regime | PUFFVOL | Puff Volume | 10 | mL | 10 | 10 | mL | |
| 16 | 123 | DU | PUFFMASTER2023 | 7 | Canadian Intense Regime | PUFFRNG | Puff Range | 100-200 | 100-200 | ||||
| 17 | 123 | DU | PUFFMASTER2023 | 8 | Canadian Intense Regime | 1 | PUFFPAUS | Puff Pause | 60 | s | 60 | 60 | s |
| 18 | 123 | DU | PUFFMASTER2023 | 9 | Canadian Intense Regime | 1 | PUFFPINT | Puff Pause Interval | 10 | PUFF | 10 | 10 | PUFF |
A1: 
A2: 
| Row | STUDYID | ASSAYID | DOMAIN | TXSETCD | GTSEQ | GTTESTCD | GTTEST | GTCELLEV (cells evaluated) | GTORRES | GTORRESU | GTSTRESC | GTSTRESN | GTSTRESU | GTDTC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 123 | MNvit | GT | A1 | 1 | RICC | Relative Increase in Cell Count | 0 | 0 | % | 0 | 0 | % | 2022-05-25 |
| 2 | 123 | MNvit | GT | A1 | 2 | RCC | Relative Cell Count | 154 | 0 | % | 0 | 0 | % | 2022-05-25 |
| 3 | 123 | MNvit | GT | A1 | 3 | RPD | Relative Population Doubling | 154 | 0 | % | 0 | 0 | % | 2022-05-25 |
| 4 | 123 | MNvit | GT | A1 | 4 | MNCELLS | Micronucleated Cells | 2205 | 15 | Cells | 15 | 15 | Cells | 2022-05-25 |
| 5 | 123 | MNvit | GT | A1 | 5 | MNCELLS | Micronucleated Cells | 2474 | 13 | Cells | 13 | 13 | Cells | 2022-05-25 |
| 6 | 123 | MNvit | GT | A1 | 6 | MNCELLS | Micronucleated Cells | 2758 | 17 | Cells | 17 | 17 | Cells | 2022-05-25 |
| 7 | 123 | MNvit | GT | A1 | 7 | MNCELLS | Micronucleated Cells | 2669 | 12 | Cells | 12 | 12 | Cells | 2022-05-25 |
| 8 | 123 | MNvit | GT | A1 | 8 | AVGREL | Average Relative MN Frequency | 0.57 | % | 0.57 | 0.57 | % | 2022-05-25 | |
| 9 | 123 | MNvit | GT | A2 | 1 | RICC | Relative Increase in Cell Count | 134 | 15.7 | % | 15.7 | 15.7 | % | 2022-05-25 |
| 10 | 123 | MNvit | GT | A2 | 2 | RCC | Relative Cell Count | 134 | 13.0 | % | 13.0 | 13.0 | % | 2022-05-25 |
| 11 | 123 | MNvit | GT | A2 | 3 | RPD | Relative Population Doubling | 134 | 7.9 | % | 7.9 | 7.9 | % | 2022-05-25 |
| 12 | 123 | MNvit | GT | A2 | 4 | MNCELLS | Micronucleated Cells | 3266 | 20 | Cells | 20 | 20 | Cells | 2022-05-25 |
| 13 | 123 | MNvit | GT | A2 | 5 | MNCELLS | Micronucleated Cells | 2190 | 17 | Cells | 17 | 17 | Cells | 2022-05-25 |
| 14 | 123 | MNvit | GT | A2 | 6 | MNCELLS | Micronucleated Cells | 2758 | 13 | Cells | 13 | 13 | Cells | 2022-05-25 |
| 15 | 123 | MNvit | GT | A2 | 7 | MNCELLS | Micronucleated Cells | 2714 | 21 | Cells | 21 | 21 | Cells | 2022-05-25 |
| 16 | 123 | MNvit | GT | A2 | 8 | AVGREL | Average Relative MN Frequency | 0.66 | % | 0.66 | 0.66 | % | 2022-05-25 | |
| ... |
- No labels