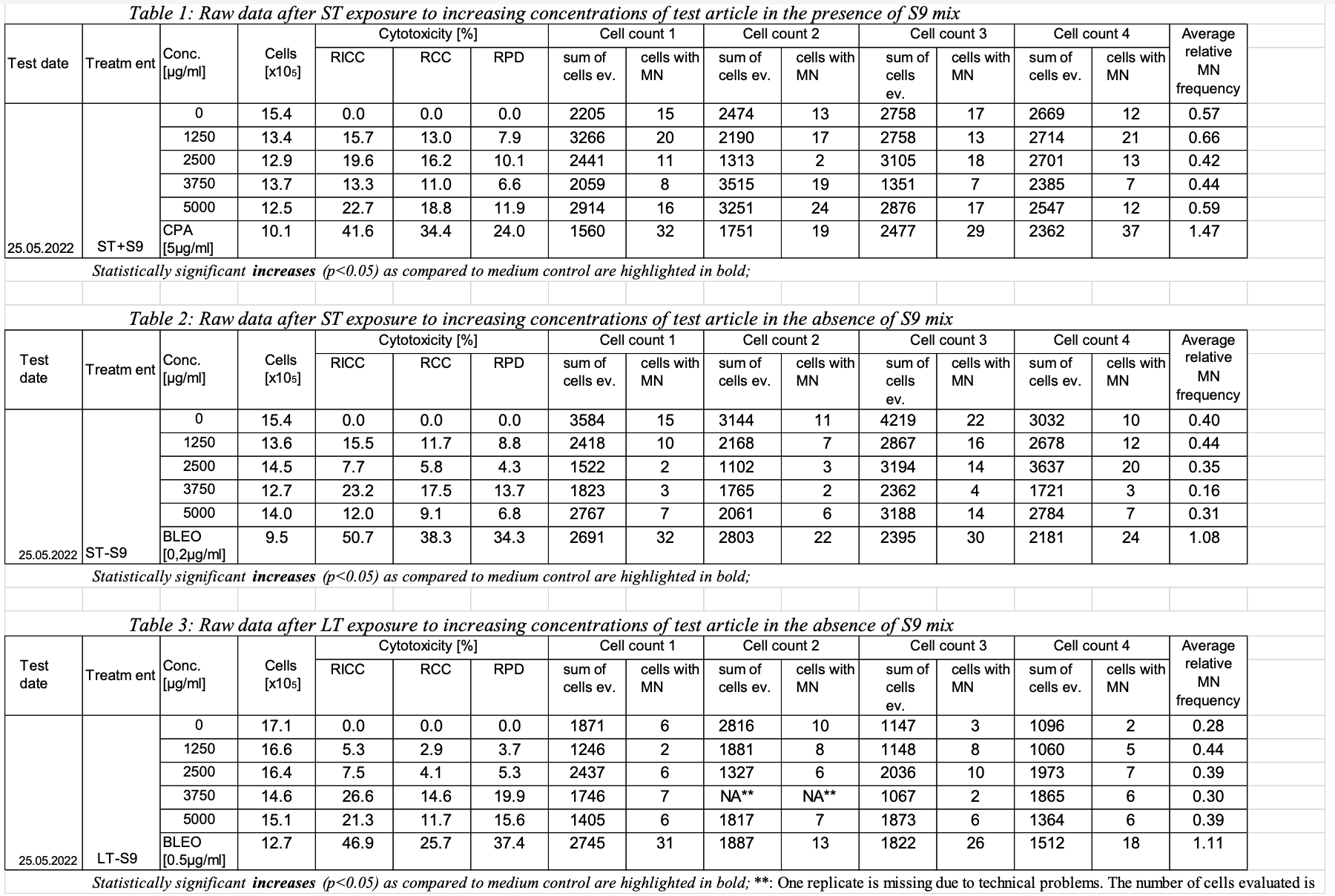
This is an example showing example shows a sample report table, trial design, and results data of Study #123 dataset for study 123 for the determination of the in vitro genotoxicity potential of 10? tobacco products in using the in vitro Micronucleus Assaymicronucleus assay.
| Expand |
|---|
| title | Sample Report Table for Study 123, Raw data (for reference only, will be deleted?) |
|---|
|
|
| Dataset wrap |
|---|
| Expand |
|---|
| title | ts.xpt (trial summary, study level parameters) |
|---|
|
| Rowcaps |
|---|
| Rows 1-2: | Show 2 records for TSPARMCD = "GLPTYP", using TSSEQ to indicate multiple records, since both GLP types apply for this example study. | | Row 3: | Shows that this study was conducted as a GLP study. | | Rows 4-5: | Show the study start date and study title. | | Rows 6-7: | Show the version of SEND Implementation Guide and version of Controlled Terminology used in this study. | | Row 8: | Shows the applicant's organization. | | Row 9: | Shows that the applicant's study reference ID is not applicable. | | Rows 10-13: | Show that TSGRPID has been used to link records (name, location, country) related to the test facility (TSGRPID = 1). The study director is associated with the test facility. | | Rows 14-16: | Show that TSGRPID (TSGRPID=2) has been used to link the information on the testing guideline followed on this study (TSTGDNAM, TSTGDORG, TSTGDVER). | | Shows the study type for this study. | | Shows that this study includes a Mammalian Cell Micronucleus Assay. | | Rows 19-20: | Show that the species is human and the cell line is TK6 lymphoblastoid in this study. |
|
- Assumption: A Trial (study) can have more than one assay type
- Assumption: ASSAYID value of ALL indicates that it applies to all assays in the study
- Assumption: SPDEVID and DUREFID should only be in TX (with the same value for all sets if there is only one device used)
- Assumption: SPDEVID (sponsor defined device identifier) should be added at the trial set level (tx.xpt). While it is possible to show a value in TS when there is only one device for a study, we will make it a rule that it goes in TX for consistency and cross-study analysis.
- This allows for studies where there are multiple products and different product(s) per trial set, one record for each product that is being tested in each particular trial set (tx.xpt)
| Row | STUDYID | ASSAYID | DOMAIN | TSSEQ | TSGRPID | TSPARMCD | TSPARM | TSVAL | TSVALNF |
|---|
| 1 | 123 |
|
MNvit | TS | 1 |
| GLPTYP | Good Laboratory Practice Type | FDA |
| | 2 | 123 |
|
MNvit | TS | 2 |
| GLPTYP | Good Laboratory Practice Type | OECD |
| | 3 | 123 |
|
MNvitSTSTDTC | Study Start Date |
| GLPFL | GLP Flag | Y |
| | 4 | 123 | TS | 1 |
| STSTDTC | Study Start Date | 2022-05-25 |
|
4MNvit | TS | 1 |
| STITLE | Study Title | Determination of the in vitro genotoxicity potential |
|
of 10 tobacco products in the in vitro Micronucleus Assayusing the in vitro Neutral Red Uptake assay |
| | 6 | 123 |
|
5 | 123 | MNvit | TS | 1 |
| SNDIGVER | SEND Implementation Guide Version | TOBACCO IMPLEMENTATION GUIDE VERSION 1.0 |
|
6MNvit | TS | 1 |
| SNDCTVER | SEND Controlled Terminology Version | SEND Terminology 2021-09-30 |
|
7MNvitSSPONSORSponsor Organization Sponsor 8MNvitSPREFIDSponsor's | Study Reference ID |
| NOT APPLICABLE |
|
9MNvit | TS | 1 | 1 | TSTFNAM | Test Facility Name | Example |
|
Tox 10MNvit | TS | 1 | 1 | TSTFLOC | Test Facility Location | 10 Somewhere Street, Montgomery, AL 10000 |
|
11MNvit | TS | 1 | 1 | TFCNTRY | Test Facility Country | USA |
|
12MNvit | TS | 1 | 1 | STDIR | Study Director | Dr. R. Smith |
|
13MNvitGLPFL | GLP Flag | Y | 14 | 123 | | TSTGDNAM | Testing Guideline Name | GUIDELINE FOR THE TESTING OF CHEMICALS No. 487 |
| | 15 | 123 |
|
MNvitASTD | Assay Standard| TSTGDORG | Testing Guideline Organization | OECD |
|
Test No. 487 15MNvitASTDV | | 2 | TSTGDVER | Testing Guideline |
|
Assay Standard 2016072916MNvit | TS | 1 |
| SSTYP | Study Type | GENOTOXICITY IN VITRO |
|
17MNvitSSSTYP | Study Sub Type | In Vitro Micronucleus |
| GNTXAID | Genetic Toxicology Assay Identifier | MNvit |
| | 19 | 123 |
|
18 | 123 | MNvitHomo Sapiens | | HUMAN |
| | 20 | 123 | TS | 1 |
| CELLLN | Cell Line | |
|
|
|---|
This example Trial Sets dataset shows information about the test conditions for set A1 and A2 in this example study. Sets A1 and A2 can be seen in the first and second rows respectively of the sample report Table 1 (above). For brevity, the TX dataset and the findings (GT) dataset do not show information for any other sets. Fully formed datasets for this example study would include information about the test conditions and findings for all sets.
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Rows 1-23: | Show trial set parameters and values that comprise the test conditions for trial set A1. Set A1 is the data for the negative control (concentration 0) with short-term exposure and metabolic activation S9. The applicant has chosen to given a long name (SET) equal to "ST+S9_C0". Set A1 is associated with the first row in the sample report table for study 123. | | Rows 24-46: | Show trial set parameters and values that comprise the test conditions for trial set A2. Set A2 is the data for the short-term exposure with metabolic activation S9 at a concentration of 1250 ug/ml. The applicant has chosen to give the set a long name (SET) equal to "ST+S9_C1250". Set A2 is associated with the second row in the sample report table for study 123. |
|
| Dataset2 |
|---|
| Row | STUDYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|
| 1 | 123 | TX | A1 | ST+S9_C0 | 1 | MTACTIND | Metabolic Activating Agent Name | +S9 | | 2 | 123 | TX | A1 | ST+S9_C0 | 2 | METACTFL | Presence of Metabolic Activation Flag | Y | | 3 | 123 | TX | A1 | ST+S9_C0 | 3 | IVTDMIN | In vitro Treatment Duration Minimum | 3 | | 4 | 123 | TX | A1 | ST+S9_C0 | 4 | IVTDTRG | In vitro |
|
|
| Expand |
|---|
|
- Assumption: SPDEVID (sponsor defined device identifier), and Test System should be added at the trial set level (tx.xpt). While it is possible to show a value in TS when there is only one device for a study, we will make it a rule that it goes in TX for consistency and cross-study analysis.
- This allows for studies where there are multiple products and different product(s) per trial set, one record for each product that is being tested in each particular trial set (tx.xpt)
- Assumption: SPDEVID and DUREFID should only be in TX (with the same value for all sets if there is only one device used)
- Assumption: SPDEVID (sponsor defined device identifier) should be added at the trial set level (tx.xpt). While it is possible to show a value in TS when there is only one device for a study, we will make it a rule that it goes in TX for consistency and cross-study analysis.
- This allows for studies where there are multiple products and different product(s) per trial set, one record for each product that is being tested in each particular trial set (tx.xpt)
A1:  Image Removed Image Removed A2:  Image Removed Image Removed |
| Row | STUDYID | ASSAYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|
| 1 | 123 | MNvit | TX | A1 (table 1, row 1, ST exposure with S9) | ST+S9C0 | 1 | TESTSYS | Test system | TK6 Lymphoblastoid Suspension Cells |
| 2 | 123 | MNvit | TX | A1 | ST+S9C0 | 2 | METACT | Metabolic Activation (this is the type of activation used) | +S9 |
| 3 | 123 | MNvit | TX | A1 | ST+S9C0 | 3 | METACTFL | Y/N presence of metabolic activation (this indicates that metabolic activation was used) | Y |
| 4 | 123 | MNvit | TX | A1 | ST+S9C0 | 4 | TRTDMIN | Treatment Duration Minimum | 3 |
5 | 123 | MNvit | TX | A1 | ST+S9C0 | 5 | TRTDTRG | | Treatment Duration Target | 3.5 |
|
6MNvitS9C06TRTDMAX| IVTDMAX | In vitro Treatment Duration Maximum | 4 |
|
7MNvitS9C07TRTDU| IVTDU | In vitro Treatment Duration Unit | HOURS |
|
8MNvitS9C08| 7 | RCVDMIN | Recovery Duration Minimum | 23.5 |
|
9MNvitS9C09| 8 | RCVDTRG | Recovery Duration Target | 24 |
|
10MNvitS9C010| 9 | RCVDMAX | Recovery Duration Maximum | 24.5 |
|
11MNvitS9C011| 10 | RCVDU | Recovery Duration Unit | HOURS |
|
12MNvitS9C012| 11 | INCBTMP | Incubation Temperature | 37 |
|
13MNvitS9C013| 12 | INCBTMPU | Incubation Temperature Unit | C |
|
14MNvitS9C014HUMID| ATMRHP | Atmospheric Relative |
|
Humidity 15MNvitS9C015ATMCO2| ATMCO2P | Atmospheric CO2 Percent | 5 |
|
16MNvitS9C016Sponsor Applicant-defined tobacco identifier | CIG01a |
|
17MNvitS9C017 18MNvitS9C018SAMTYP | SAMTYP | Sample Type | Total Particulate Matter in DMSO |
|
19MNvitS9C019INTRVNName of the Intervention Article Name | Tobacco ProdA |
|
20MNvitS9C020choices of values: product; negative control; positive controltype of intervention article
Intervention Article Type | Negative Control |
|
21MNvitS9C021| 20 | ITVCONC | Intervention Article Concentration |
|
of intervention article22MNvitS9C022| 21 | ITVCONCU | Intervention Article Concentration Unit | ug/ml |
|
23MNvitS9C023Sponsor | Applicant-defined device identifier | PUFFMASTER3K |
|
24MNvitS9C024DUREFID Smoke Medium Intensity Regimen | | MEDIUM INTENSITY REGIMEN | | 24 |
|
25MNvit(table 1, row 2, ST exposure with S9 at concentration 1250)
| ST+S9_C1250 | 24 | MTACTIND | Metabolic Activating Agent Name | +S9 | | 25 | 123 | TX | A2 | ST+S9_C1250 | 25 | METACTFL | Presence of Metabolic Activation Flag | Y | | 26 | 123 |
|
ST+S9-1250 | 1 | TESTSYS | Test system | TK6 Lymphoblastoid Suspension Cells | 26 | 123 | MNvit-12502METACT | Metabolic Activation (this is the type of activation used) | IVTDMIN | In vitro Treatment Duration Minimum | 3 |
|
+S9MNvit-12503METACTFL | Y/N presence of metabolic activation (this indicates that metabolic activation was used) | | IVTDTRG | In vitro Treatment Duration Target | 3.5 |
|
YMNvit-12504TRTDMIN| IVTDMAX | In vitro Treatment Duration |
|
Minimum3MNvit-12505TRTDTRG| IVTDU | In vitro Treatment Duration |
|
Target3.5MNvit-12506TRTDMAXTreatment Maximum4MNvit-12507TRTDUTreatment UnitHOURSMNvit-12508RCVDMIN Minimum23MNvit-12509RCVDTRG Target24MNvit-125010RCVDMAX | Recovery Duration Maximum | | INCBTMP | Incubation Temperature | 37 |
|
24.5MNvit-125011RCVDURecovery Duration | Incubation Temperature Unit |
|
HOURSMNvit-125012INCBTMP | Incubation Temperature | | ATMRHP | Atmospheric Relative Humidity Percent | 50 |
|
37MNvit-125013INCBTMPU | Incubation Temperature Unit | | ATMCO2P | Atmospheric CO2 Percent | 5 |
|
CMNvit | -125014HUMID | Atmospheric Relative Humidity Percent | SPTOBID | Applicant-defined tobacco identifier | CIG01a |
|
50MNvit-125015ATMCO2 | Atmospheric CO2 Percent | 5MNvit-125016SPTOBID | Sponsor defined tobacco identifier | | SAMTYP | Sample Type | Total Particulate Matter in DMSO |
|
CIG01aMNvit-125017EXPTYP | Exposure Type (See TIG NC workstream minutes 30-Jan here: Nonclinical) | | ITVNAM | Intervention Article Name | Tobacco ProdA |
|
SubmergedMNvit-125018SAMTYP Sample Type (e.g. TPM, GVP, whole aerosol, whole smoke conditioned media, aqueous extracts, etc., see notes here: Nonclinical) | Total Particulate Matter in DMSO | Intervention Article Type | Product | | 43 | 123 | TX | A2 | ST+S9_C1250 | 43 | ITVCONC | Intervention Article Concentration | 1250 | | 44 | 123 |
|
43 | 123 | MNvit-125019INTRVNName of the Tobacco ProdA | | Concentration Unit | ug/ml | | 45 |
|
44MNvit-125020ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Product | | SPDEVID | Applicant-defined Device Identifier | PUFFMASTER2023 | | 46 | 123 |
|
45 | 123 | MNvit-125021ITVCONC | Concentration of intervention article | 1250 | | 46 | 123 | MNvit | TX | A2 | ST+S9-1250 | 22 | ITVCONCU | Concentration Unit | ug/ml |
| 47 | 123 | MNvit | TX | A2 | ST+S9-1250 | 23 | SPDEVID | Sponsor defined device identifier | PUFFMASTER2023 |
| 48 | 123 | MNvit | TX | A2 | ST+S9-1250 | 24 | DUREFID | Smoke Regimen | High Intensity Regimen |
... | | Expand |
|---|
| title | to.xpt (tobacco identifiers, design paramters) |
|---|
|
(This is a copy from the SDTM Example. Cigarette Design Parameters)
| Rows 1-4: | Show the records for the product identifiers for the tobacco product identified in SPTOBID. These records are categorized as product identifiers by TOCAT = PRODUCT IDENTIFIERS. |
|---|
| Rows 5-10: | Show the records for the product descriptors for the tobacco product identified in SPTOBID. These records are categorized as product identifiers by TOCAT = PRODUCT DESCRIPTOR. |
|---|
to.xpt
Row | STUDYID | DOMAIN | SPTOBID | TOSEQ | TOTESTCD | TOTEST | TOCAT | TOORRES | TOORRESU | TOSTRESC | TOSTRESN | TOSTRESU |
|---|
1 | TOB07 | TO | CIG01a | | TBPRDCAT | Tobacco Product Category | PRODUCT IDENTIFIER | Cigarette | Cigarette | 2 | TOB07 | TO | CIG01a | 2 | TBPRSCAT | Tobacco Product Subcategory | PRODUCT IDENTIFIER | Filtered, Combusted | Filtered, Combusted | 3 | TOB07 | TO | CIG01a | 3 | MANUF | Manufacturer | PRODUCT IDENTIFIER | Joes Cigs USA | Joes Cigs USA | 4 | TOB07 | TO | CIG01a | 4 | TRADENAM | Trade Name | PRODUCT IDENTIFIER | Treetop Menthol King Size | Treetop Menthol King Size | SMKRGM | Smoking Regimen | HIGH INTENSITY REGIMEN |
|
|
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Row 1: | Shows the value of REFID=C0. This REFID refers to the trial set with a SETCD of "A1", as defined in the TX dataset. LEVEL=1 and LVLDESC="EXPERIMENTAL UNIT/TRIAL SET" indicates this identifier is referring to both the experimental unit and the unit to which the treatment is applied, and to the entire trial set. | | Rows 2-5: | Show the values of 4 observational units (C0_Count1 through C0_Count4) that are within the parent experimental unit, REFID=C0. In this example assay, these observational units are also all within the same trial set, as defined in the TX dataset. | | Row 6: | Shows the value of REFID=C1250. This REFID refers to the trial set with a SETCD of "A2", as defined in the TX dataset. LEVEL=1 and LVLDESC="EXPERIMENTAL UNIT/TRIAL SET" indicates this identifier is referring to both the experimental unit and the unit to which the treatment is applied, and to the entire trial set. | | Rows 7-10: | Show the values of 4 observational units (C1250_Count1 through C1250_Count4) that are within the parent experimental unit, REFID=C1250. In this example assay, these observational units are also all within the same trial set, as defined in the TX dataset. |
|
| Dataset2 |
|---|
| Row | STUDYID | SETCD | REFID | PARENT | LEVEL | LVLDESC |
|---|
1 | 123 | | C0 |
| 1 | EXPERIMENTAL UNIT/TRIAL SET | 2 | 123 | A1 | C0-Count1 | C0 | 2 | OBSERVATIONAL UNIT | 3 | 123 | A1 | C0-Count2 | C0 | 2 | OBSERVATIONAL UNIT | 4 | 123 | A1 | C0-Count3 | C0 | 2 | OBSERVATIONAL UNIT | 5 | 123 | A1 | C0-Count4 | C0 | 2 | OBSERVATIONAL UNIT | 6 | 123 | A2 | C1250 |
| 1 | EXPERIMENTAL UNIT/TRIAL SET | | 7 | 123 | A2 | C1250-Count1 | C1250 | 2 | OBSERVATIONAL UNIT | | 8 | 123 | A2 | C1250-Count2 | C1250 | 2 | OBSERVATIONAL UNIT | | 9 | 123 | A2 | C1250-Count3 | C1250 | 2 | OBSERVATIONAL UNIT | | 10 | 123 | A2 | C1250-Count4 | C1250 | 2 | OBSERVATIONAL UNIT |
|
|
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
Rows 1-3, 8: | Show percentage result values that apply to GTREFID=C0. REFID=C0, as shown in the RELREF dataset, relates this data to the trial set in the first row of table 1 in the sample report table for study 123. | | Rows 4-7: | Show the 4 micronucleated cell counts for the observational units with GTREFID from C0-Count1 through C0-Count4, for which their relationship to test conditions (in tx.xpt) and experimental units (in relref.xpt) are shown in the RELREF dataset. | Rows 9-11, 16: | Show percentage result values that apply to GTREFID=C1250. REFID=C1250, as shown in the RELREF dataset, relates this data to the trial set in the second row of table 1 in the sample report table for study 123. | | Rows 12-15: | Show the 4 micronucleated cell counts for the observational units with GTREFID from C1250-Count1 through C1250-Count4, for which their relationship to test conditions (in tx.xpt) and experimental units (in relref.xpt) are shown in the RELREF dataset. |
|
| Dataset2 |
|---|
| Row | STUDYID | DOMAIN | GTSEQ | GTREFID | GTTESTCD | GTTEST | GTCELLEV | GTORRES | GTORRESU | GTCOLSRT |
|---|
|
|
5 | TOB07 | TO | CIG01a | 5 | PACKTYP | Package Type | PRODUCT DESCRIPTOR | HARD PACK | HARD PACK | | 6 | TOB07 | TO | CIG01a | 6 | PRDQUAN | Product Quantity | PRODUCT DESCRIPTOR | 20 | CIGARETTE | 20 | 20 | CIGARETTE |
|---|
| 7 | TOB07 | TO | CIG01a | 7 | LENGTH | Length | PRODUCT DESCRIPTOR | 86.0 | mm | 86.0 | 86.0 | mm |
|---|
| 8 | TOB07 | TO | CIG01a | 8 | CIRCUMF | Circumference | PRODUCT DESCRIPTOR | 26.0 | mm | 26.0 | 26.0 | mm |
|---|
| 9 | TOB07 | TO | CIG01a | 9 | VENTLTN | Ventilation | PRODUCT DESCRIPTOR | 10.0 | % | 10.0 | 10.0 | % |
|---|
10 | TOB07 | TO | CIG01a | 10 | CHARFLAV | Characterizing Flavor | PRODUCT DESCRIPTOR | MENTHOL | MENTHOL | | Expand |
|---|
| title | di.xpt (a study reference domain for unique device identification) |
|---|
|
di.xpt (copied v1.0 of medical devices IG)
- This example is a copy of Example 1 from di.xpt in SDTMIG-MD v1.0 but with values for SPDEVID and DIVAL revised slightly.
- Should I remove the FDA UDI (row 5) unless CTP has or plans to establish UDI values?
Example 1
This shows records for two three devices where the sponsor felt that the type, manufacturer, model number, and serial number were necessary for unique identification. In addition, there was a post-marketing UDI identifier available for the first device.
- Rows 1-5 show the records for a device given a SPDEVID of PUFFMASTER3K
- Rows 5-8 show the records for a device given a SPDEVID of PUFFMASTER2023
- Rows 9-12 show
Row | STUDYID | DOMAIN | SPDEVID | DISEQ | DIPARMCD | DIPARM | DIVAL |
1 | 123 | DI | PUFFMASTER3K | 1 | DEVTYPE | Device Type | ENDS |
2 | 123 | DI | PUFFMASTER3K | 2 | MANUF | Manufacturer | Acme Machines |
3 | 123 | DI | PUFFMASTER3K | 3 | MODEL | Model Number | 45-JFI |
4 | 123 | DI | PUFFMASTER3K | 4 | SERIAL | Serial Number | 456789132-AXQ |
5 | 123 | DI | PUFFMASTER3K | 5 | FDAUDI | FDA Unique Device Identifier | 456789123xyz |
6 | 123 | DI | PUFFMASTER2023 | 1 | DEVTYPE | Device Type | SmokeMachine |
7 | 123 | DI | PUFFMASTER2023 | 2 | MANUF | Manufacturer | Acme Machines |
8 | 123 | DI | PUFFMASTER2023 | 3 | MODEL | Model Number | 62-PLC |
9 | 123 | DI | PUFFMASTER2023 | 4 | SERIAL | Serial Number | 215964564-NFS |
10 | 123 | DI | UsualCTCigarette | 1 | DEVTYPE | Device Type | Combustible Tobacco Cigarette |
11 | 123 | DI | UsualCTCigarette | 2 | MANUF | Manufacturer | Philip Morris International |
12 | 123 | DI | UsualCTCigarette | 3 | MODEL | Model Number | Marlboro Red |
13 | 123 | DI | UsualCTCigarette | 4 | SERIAL | Serial Number | 123456789 |
| Expand |
|---|
| title | du.xpt smoke regimen definition (a findings domain for device in-use properties) |
|---|
|
We use DU for smoking regimen. Note that a separate DI dataset will be needed to show identifying parameters of the "PUFFMASTER3K" smoking machine
- Details of the smoking regimen are represented as device in-use properties, linked to the stability data in PT above by matching values of PTREFID/DUREFID = "Medium Intensity Regimen" (We will update with a realistic value for the regimen, with input).
- Smoking regimen is represented in --REFID (we made up a value of "Medium Intensity Regimen"; we can update with something realistic)
- The smoking regimen is carried out by the smoking machine/device shown in SPDEVID, Sponsor defined device identifier, "PUFFMASTER3K"
du.xpt
| STUDYID | DOMAIN | SPDEVID | DUSEQ | DUREFID | DUGRPID | DUTESTCD | DUTEST | DUORRES | DUORRESU | DUSTRESC | DUSTRESN | DUSTRESU |
|---|
1 | 123 | DU | PUFFMASTER3K | 1 | Medium Intensity Regimen | PUFFPROF | Puff Profile | SQUARE | SQUARE | 2 | 123 | DU | PUFFMASTER3K | 2 | Medium Intensity Regimen | PUFFDUR | Puff Duration | 1.25 | sec | 1.25 | 1.25 | sec | 3 | 123 | DU | PUFFMASTER3K | 3 | Medium Intensity Regimen | PUFFINT | Puff Interval | 3 | PUFF/min | 3 | 3 | PUFF/min | 4 | 123 | DU | PUFFMASTER3K | 4 | Medium Intensity Regimen | PUFFBLCK | Puff Block | 25 | % | 25 | 25 | % | 5 | 123 | DU | PUFFMASTER3K | 5 | Medium Intensity Regimen | NUMPUFF | Total Number of Puffs | 200 | PUFF | 200 | 200 | PUFF | 6 | 123 | DU | PUFFMASTER3K | 6 | Medium Intensity Regimen | PUFFVOL | Puff Volume | 10 | mL | 10 | 10 | mL | 7 | 123 | DU | PUFFMASTER3K | 7 | Medium Intensity Regimen | PUFFRNG | Puff Range | 100-200 | 100-200 | | 8 | 123 | DU | PUFFMASTER3K | 8 | Medium Intensity Regimen | 1 | PUFFPAUS | Puff Pause | 60 | s | 60 | 60 | s |
|---|
| 9 | 123 | DU | PUFFMASTER3K | 9 | Medium Intensity Regimen | 1 | PUFFPINT | Puff Pause Interval | 10 | PUFF | 10 | 10 | PUFF |
|---|
10 | 123 | DU | PUFFMASTER2023 | 1 | Canadian Intense Regime | PUFFPROF | Puff Profile | SQUARE | SQUARE | 11 | 123 | DU | PUFFMASTER2023 | 2 | Canadian Intense Regime | PUFFDUR | Puff Duration | 2.00 | sec | 2.00 | 2.00 | sec | 12 | 123 | DU | PUFFMASTER2023 | 3 | Canadian Intense Regime | PUFFINT | Puff Interval | 4 | PUFF/min | 4 | 4 | PUFF/min | 13 | 123 | DU | PUFFMASTER2023 | 4 | Canadian Intense Regime | PUFFBLCK | Puff Block | 0 | % | 0 | 0 | % | 14 | 123 | DU | PUFFMASTER2023 | 5 | Canadian Intense Regime | NUMPUFF | Total Number of Puffs | 200 | PUFF | 200 | 200 | PUFF | 15 | 123 | DU | PUFFMASTER2023 | 6 | Canadian Intense Regime | PUFFVOL | Puff Volume | 10 | mL | 10 | 10 | mL | 16 | 123 | DU | PUFFMASTER2023 | 7 | Canadian Intense Regime | PUFFRNG | Puff Range | 100-200 | 100-200 | | 17 | 123 | DU | PUFFMASTER2023 | 8 | Canadian Intense Regime | 1 | PUFFPAUS | Puff Pause | 60 | s | 60 | 60 | s |
|---|
| 18 | 123 | DU | PUFFMASTER2023 | 9 | Canadian Intense Regime | 1 | PUFFPINT | Puff Pause Interval | 10 | PUFF | 10 | 10 | PUFF |
|---|
| Expand |
|---|
| title | ei.xpt Entity Identifiers (dm-like) |
|---|
|
| Row | STUDYID | ASSAYID | DOMAIN | SETCD (from TX) | EUID | OBUID |
|---|
1 | 123 | MNvit | EI | A1 | 1-8-3 | 2 | 123 | MNvit | EI | A1 | 2-8-3 | 3 | 123 | MNvit | EI | A1 | 2-9-3 | 4 | 123 | MNvit | EI | A2 | 3-7-1 | 5 | 123 | MNvit | EI | A2 | 2-7-1 | | Expand |
|---|
| title | gt.xpt (similar to LB) |
|---|
|
A1:  Image Removed Image Removed A2:  Image Removed Image Removed - Different runs would be expected to have different ENID and GTDTC values. In ei.xpt, these are distinguished by different RUNID values.
| Row | STUDYID | ASSAYID | DOMAIN | EUID | OBUID ?? | GTSEQ | GTTESTCD | GTTEST | GTCELLEV
(cells evaluated) | GTORRES | GTORRESU | GTCOLSRT
??? | GTSTRESC | GTSTRESN | GTSTRESU | GTDTC |
|---|
| 1 | 123 |
|
MNvit-8-31| C0 | | Relative Increase in Cell Count | 154 | 0 | % |
| 0 | 0 | % | 2022-05-25 | | 2 | 123 |
|
MNvit1-8-3 | 2 | RCC | Relative Cell Count | 154 | 0 | % |
| 0 | 0 | % | 2022-05-25 | | 3 | 123 |
|
MNvit1-8-3| C0 | RPD | Relative Population Doubling | 154 | 0 | % |
| 0 | 0 | % | 2022-05-25 | | 4 | 123 |
|
MNvitGT18-34 | MNCELLS| Count1 | MNCE | Micronucleated Cells | 2205 | 15 |
|
CellsCellsMNvit18-35 | MNCELLS | Micronucleated Cells | 2474 | 13 |
|
CellsCellsMNvitGT18-36 | MNCELLS | Micronucleated Cells | 2758 | 17 |
|
CellsCellsMNvitGT18-37 | MNCELLS | Micronucleated Cells | 2669 | 12 |
|
CellsCellsMNvit1--38AVGREL | Average Relative MN Frequency| MNCECE | Micronucleated Cells/Total Cells |
| 0.57 | % |
| 0.57 | 0.57 | % | 2022-05-25 | | 9 | 123 |
|
MNvit2-8-3 | 1 | RICC | Relative Increase in Cell Count | 134 | 15.7 | % |
| 15.7 | 15.7 | % | 2022-05-25 | | 10 | 123 |
|
MNvit-8-32| C1250 | RCC | Relative Cell Count | 134 | 13.0 | % |
| 13.0 | 13.0 | % | 2022-05-25 | | 11 | 123 |
|
MNvit2-8-3| C1250 | RPD | Relative Population Doubling | 134 | 7.9 | % |
| 7.9 | 7.9 | % | 2022-05-25 | | 12 | 123 |
|
MNvitGT28-34 | MNCELLS | Micronucleated Cells | 3266 | 20 |
|
CellsCellsMNvitGT28-35 | MNCELLS | Micronucleated Cells | 2190 | 17 |
|
CellsCellsMNvitGT28-36 | MNCELLS | Micronucleated Cells | 2758 | 13 |
|
CellsCellsMNvitGT28-37 | MNCELLS | Micronucleated Cells | 2714 | 21 |
|
CellsCellsMNvit2--38AVGREL | | MNCECE | Micronucleated Cells/Total Cells |
|
Average Relative MN Frequency...
|---|


