Timeframes, fixed points in time, and other milestones may be defined in the context of a study as references to which the timing of other collected observations is related. In such cases, timeframes are referred to as reference periods and such fixed points in time and milestones are referred to as reference points.
Timing Relative to Reference Periods and Other Dates
The start and end dates (and times when applicable) of an applicant-defined reference period for a study are represented using variables RFSTDTC and RFENDTC, respectively, in the Demographics (DM) domain. Applicants may also define dates/times, in addition to a study reference period, from which to reference the relative timing of observations. In both cases, such dates are used as references from which to populate the variables described below.
In general observation class domains, days relative to the reference start date or in relation to applicant-defined reference dates
for observations
The SDTM allows for the representation of study days relative to the RFSTDTC reference start date variable in the DM dataset, using variables --DY, as described in Section 4.4.4, Use of the "Study Day" Variables. The calculation of additional study days within subdivisions of time in a clinical trial may be based on 1 or more sponsor-defined reference dates not represented by RFSTDTC. In such cases, the sponsor may define supplemental qualifier variables and the Define-XML document should reflect the reference dates used to calculate such study days. If the sponsor wishes to define "day within element" or "day within epoch", the reference date/time will be an element start date/time in the Subject Elements (SE) dataset (see Section 5.3, Subject Elements).
in general observation class domains?
| Metadataspec |
|---|
| Num | Variable Population | Implementation |
|---|
| 1 | Days Relative to the Reference Start Daterelative to a reference start date | Variables --DY, --STDY, and --ENDY represent the timing of observations in days relative to the reference start date in RFSTDTC where the value of: - --DY is the relative day of the date populated in --DTC for the observation,
- --STDTC is the relative day of the date populated in --STDTC for the observation; , and
- --ENDY is the relative day of the of the date populated in --ENDTC for the observation.
All relative day values will be integers and there will be no study day 0. The reference start date is considered day 1 and the study day value is increased by 1 for each date following the reference start date and decreased by 1 for each date prior to the reference start date, e.g.: --DY = (date portion of --DTC) - (date portion of RFSTDTC) + 1 if --DTC is on or after RFSTDTC
--DY = (date portion of --DTC) - (date portion of RFSTDTC) if --DTC precedes RFSTDTC
| | 2 | Days Relative relative to Applicant-Defined Reference Datesother applicant-defined reference dates | Days may be represented relative to other Applicants may represent days relative to applicant-defined reference dates beyond a reference period for a study. In such cases: - Relative days will be represented in a nonstandard variable (NSV) in a --SUPP dataset associated with the domain.
- All relative day values Values will be integers and there will be no day 0.
- The reference date will be day 1.Relative days will be represented in a nonstandard variable (NSV) in a --SUPP dataset associated with the domain. is considered day 1 and the relative day value is increased by 1 for each date following the reference date and decreased by 1 for each date prior to the reference date.
| | 3 | Timing of observation start relative to the reference period | - Variable --STRF represents the timing of observations relative to an applicant-defined reference period represented in RFSTDTC and RFENDTC, when relative timing such as "BEFORE", "PRIOR", "ONGOING"', or "CONTINUING" is collected instead of an actual date and is collected in relation to the applicant-defined study reference period.
- Variable --STRF is used to identify the start of an observation relative to the applicant-defined study reference period.
- Allowable values for --STRF are "BEFORE", "DURING", "DURING/AFTER", "AFTER", and "UNKNOWN". Although "COINCIDENT" and "ONGOING" are in the STENRF codelist, they describe timing relative to a point in time rather than an interval of time, so are not appropriate for use with --STRF variables. It would be unusual for an event or intervention to be recorded as starting "AFTER" the study reference period, but could be possible, depending on how the study reference period is defined in a particular study.
| | 4 | Timing of observation end relative to the reference period | - Variable --ENRF represents the timing of observations relative to an applicant-defined reference period represented in RFSTDTC and RFENDTC, when relative timing such as "BEFORE", "PRIOR", "ONGOING"', or "CONTINUING" is collected instead of an actual date and is collected in relation to the applicant-defined study reference period.
- Variable --ENRF is used to identify the end of an observation relative to the applicant-defined study reference period.
- Allowable values for --ENRF are "BEFORE", "DURING", "DURING/AFTER", "AFTER" and "UNKNOWN". If --ENRF is used, then --ENRF = "AFTER" means that the event did not end before or during the study reference period. Although "COINCIDENT" and "ONGOING" are in the STENRF codelist, they describe timing relative to a point in time rather than an interval of time, so are not appropriate for use with --ENRF variables.
|
|
Timing Relative to Reference Points
Applicants may define fixed reference points within a study from which to reference the relative timing of observations. In such cases, timepoints are used as references from which to populate the variables described below.
| Metadataspec |
|---|
| Num | Variable Population | Implementation |
|---|
| 1 | Observation start timing relative to a fixed reference point
| - Variable --STTPT represents an applicant-defined fixed reference point that characterizes the start of an observation. Allowable values for --STTPT are a description of or the date and/or time of the fixed reference time point.
- Variable --STRTPT represents the start of an observation relative to a reference time point represented in --STTPT. Allowable values are that an observation can:
- start "BEFORE" the reference point,
- start "AFTER" the reference point,
- start "COINCIDENT" with the reference point, or
- be "UNKNOWN" when it started.
| | 2 | Observation end timing relative to a fixed reference point | - Variable --ENTPT represents an applicant-defined fixed reference point that characterizes the end of an observation. Allowable values for --ENTPT are a description of or the date and/or time of the fixed reference time point.
- Variable --ENRTPT represents the start of an observation relative to a reference time point represented in --ENTPT. Allowable values are that an observation can:
- end "BEFORE" the reference point,
- end "COINCIDENT" with the reference point,
- end "AFTER" the reference point,
- be known that it did not end but was "ONGOING", or
- be "UNKNOWN" when it ended or if it was ongoing.
| | 3 | Planned intervals relative to a fixed reference point | When instances of an activity are scheduled at planned time intervals relative to a fixed reference point (e.g., an exposure), the following timing variables will be used: | Variable | Description of Use |
|---|
| --TPTREF | Represents a description of the fixed reference point from which the planned observation will be made | | --RFTDTC | Represents the actual date/time of the value of --TPTREF | | --ELTM | Represents the planned elapsed time from the fixed timepoint reference to the planned observation. The value of this variable is usually also reflected in the value of variable --TPT. | | --TPT | Represents the label for the time point relative to the value of --TPTREF | | --TPTNUM | Represents the order of time points represented in --TPT relative to the value of --TPTREF |
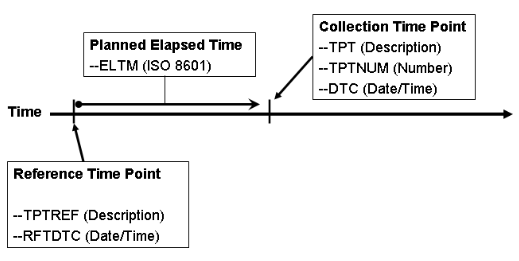 Image Added Image Added
|
|
