| Dataset wrap |
|---|
|
| Rowcaps |
|---|
Rows 1-3, 8: | Show percentage result values that apply to GTREFID=C0. REFID=C0, as shown in the RELREF dataset, relates this data to the trial set in the first row of table 1 in the sample report table for study 123. | | Rows 4-7: | Show the 4 micronucleated cell counts for the observational units with GTREFID from C0-Count1 through C0-Count4, for which their relationship to test conditions (in tx.xpt) and experimental units (in relref.xpt) are shown in the RELREF dataset. | Rows 9-11, 16: | Show percentage result values that apply to GTREFID=C1250. REFID=C1250, as shown in the RELREF dataset, relates this data to the trial set in the second row of table 1 in the sample report table for study 123. | | Rows 12-15: | Show the 4 micronucleated cell counts for the observational units with GTREFID from C1250-Count1 through C1250-Count4, for which their relationship to test conditions (in tx.xpt) and experimental units (in relref.xpt) are shown in the RELREF dataset. |
|
| Dataset2 |
|---|
| Row | STUDYID | DOMAIN | GTSEQ | GTREFID | GTTESTCD | GTTEST | GTCELLEV | GTORRES | GTORRESU | GTCOLSRT |
|---|
|
|
MNvit | TX | A1 (table 1, row 1, ST exposure with S9) | METACT | Metabolic Activation (should there be two parms? Presence, type)? | +S9 | METACTFL | Y/N presence of metabolic activation | 123 | MNvit | TX | A1 | TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) | 3 | 123 | MNvit | TX | A1 | TRTDRTOL | Treatment Duration Tolerance | 123 | MNvit | TX | A1 | TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | H | 123 | MNvit | TX | A1 | INTRVN | name of the intervention article (Tobacco ProdA, Bleomycin or Cyclophosphamid A) | Tobacco ProdA | 123 | MNvit | TX | A1 | ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Product | 123 | MNvit | TX | A1 | ITVCONC | Concentration of intervention article | 0 | 123 | MNvit | TX | A1 | ITVCONCU | Concentration Unit | ug/ml | 123 | MNvit | TS | A1 | SPDEVID | Sponsor defined device identifier | PUFFMASTER3K | 123 | MNvit | TS | A1 | DUREFID | Smoke Regimen | Medium Intensity Regimen | 123 | MNvit | TX | A2 (table 1, row 2) | METACT | Metabolic Activation (should there be two parms? Presence, type)? | +S9 | 123 | MNvit | TX | A2 | TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) | 3 | 123 | MNvit | TX | A2 | TRTDRTOL | Treatment Duration Tolerance | 123 | MNvit | TX | A2 | TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | H | 123 | MNvit | TX | A2 | INTRVN | name of the intervention article | Tobacco ProdA | 123 | MNvit | TX | A2 | ITVTYPE | type of intervention article | Product | 123 | MNvit | TX | A2 | ITVCONC | Concentration of i a | 1250 | 123 | MNvit | TX | A2 | ITVCONCU | Concentration Unit | ug/ml | 123 | MNvit | TS | A2 | SPDEVID | Sponsor defined device identifier | PUFFMASTER2023 | 123 | MNvit | TS | A2 | DUREFID | Smoke Regimen | High Intensity Regimen | ... | | Expand |
|---|
| title | du.xpt (device in-use properties) |
|---|
|
We use DU for smoking regimen. Note that a separate DI dataset will be needed to show identifying parameters of the "PUFFMASTER3K" smoking machine, but is currently not shown for brevity.
- Details of the smoking regimen are represented as device in-use properties, linked to the stability data in PT above by matching values of PTREFID/DUREFID = "Medium Intensity Regimen" (We will update with a realistic value for the regimen, with input).
- Smoking regimen is represented in --REFID (we made up a value of "Medium Intensity Regimen"; we can update with something realistic)
- The smoking regimen is carried out by the smoking machine/device shown in SPDEVID, Sponsor defined device identifier, "PUFFMASTER3K"
- Do we need both SPDEVID AND DUREFID?
du.xpt
| STUDYID | DOMAIN | SPDEVID | DUSEQ | DUREFID | DUGRPID | DUTESTCD | DUTEST | DUORRES | DUORRESU | DUSTRESC | DUSTRESN | DUSTRESU |
|---|
1 | 123 | DU | PUFFMASTER3K | 1 | Medium Intensity Regimen | PUFFPROF | Puff Profile | SQUARE | SQUARE | 2 | 123 | DU | PUFFMASTER3K | 2 | Medium Intensity Regimen | PUFFDUR | Puff Duration | 1.25 | sec | 1.25 | 1.25 | sec | 3 | 123 | DU | PUFFMASTER3K | 3 | Medium Intensity Regimen | PUFFINT | Puff Interval | 3 | PUFF/min | 3 | 3 | PUFF/min | 4 | 123 | DU | PUFFMASTER3K | 4 | Medium Intensity Regimen | PUFFBLCK | Puff Block | 25 | % | 25 | 25 | % | 5 | 123 | DU | PUFFMASTER3K | 5 | Medium Intensity Regimen | NUMPUFF | Total Number of Puffs | 200 | PUFF | 200 | 200 | PUFF | 6 | 123 | DU | PUFFMASTER3K | 6 | Medium Intensity Regimen | PUFFVOL | Puff Volume | 10 | mL | 10 | 10 | mL | 7 | 123 | DU | PUFFMASTER3K | 7 | Medium Intensity Regimen | PUFFRNG | Puff Range | 100-200 | 100-200 | | 8 | 123 | DU | PUFFMASTER3K | 8 | Medium Intensity Regimen | 1 | PUFFPAUS | Puff Pause | 60 | s | 60 | 60 | s |
|---|
| 9 | 123 | DU | PUFFMASTER3K | 9 | Medium Intensity Regimen | 1 | PUFFPINT | Puff Pause Interval | 10 | PUFF | 10 | 10 | PUFF |
|---|
10 | 123 | DU | PUFFMASTER2023 | 1 | Canadian Intense Regime | PUFFPROF | Puff Profile | SQUARE | SQUARE | 11 | 123 | DU | PUFFMASTER2023 | 2 | Canadian Intense Regime | PUFFDUR | Puff Duration | 2.00 | sec | 2.00 | 2.00 | sec | 12 | 123 | DU | PUFFMASTER2023 | 3 | Canadian Intense Regime | PUFFINT | Puff Interval | 4 | PUFF/min | 4 | 4 | PUFF/min | 13 | 123 | DU | PUFFMASTER2023 | 4 | Canadian Intense Regime | PUFFBLCK | Puff Block | 0 | % | 0 | 0 | % | 14 | 123 | DU | PUFFMASTER2023 | 5 | Canadian Intense Regime | NUMPUFF | Total Number of Puffs | 200 | PUFF | 200 | 200 | PUFF | 15 | 123 | DU | PUFFMASTER2023 | 6 | Canadian Intense Regime | PUFFVOL | Puff Volume | 10 | mL | 10 | 10 | mL | 16 | 123 | DU | PUFFMASTER2023 | 7 | Canadian Intense Regime | PUFFRNG | Puff Range | 100-200 | 100-200 | | 17 | 123 | DU | PUFFMASTER2023 | 8 | Canadian Intense Regime | 1 | PUFFPAUS | Puff Pause | 60 | s | 60 | 60 | s |
|---|
| 18 | 123 | DU | PUFFMASTER2023 | 9 | Canadian Intense Regime | 1 | PUFFPINT | Puff Pause Interval | 10 | PUFF | 10 | 10 | PUFF |
|---|
| Expand |
|---|
di.xpt (copied v1.0 of medical devices IG)
- This example is a copy of Example 1 from di.xpt in SDTMIG-MD v1.0 but with values for SPDEVID and DIVAL revised slightly.
- Should I remove the FDA UDI (row 5) unless CTP has or plans to establish UDI values?
Example 1
This shows records for two devices where the sponsor felt that the type, manufacturer, model number, and serial number were necessary for unique identification. In addition, there was a post-marketing UDI identifier available for the first device.
- Rows 1-5 show the records for a device given a SPDEVID of ABC001
- Rows 5-8 show the records for a device given a SPDEVID of ABC999
Row | STUDYID | DOMAIN | SPDEVID | DISEQ | DIPARMCD | DIPARM | DIVAL |
1 | 2011-001 | DI | PUFFMASTER3K | 1 | DEVTYPETYPE | Device Type | MRIAll values in DIVAL corresponding to DEVTYPE in all examples need to be updated to conform with FDA Product Classification codes |
2 | 2011-001 | DI | PUFFMASTER3K | 2 | MANUF | Manufacturer | Acme Machines |
3 | 2011-001 | DI | PUFFMASTER3K | 3 | MODEL | Model Number | 45-JFI |
4 | 2011-001 | DI | PUFFMASTER3K | 4 | SERIAL | Serial Number | 456789132-AXQ |
5 | 2011-001 | DI | PUFFMASTER3K | 5 | FDAUDI | FDA Unique Device Identifier | 456789123xyz |
6 | 2015-001 | DI | PUFFMASTER2023 | 1 | DEVTYPETYPE | Device Type | MRI |
7 | 2015-001 | DI | PUFFMASTER2023 | 2 | MANUF | Manufacturer | Acme Machines |
8 | 2015-001 | DI | PUFFMASTER2023 | 3 | MODEL | Model Number | 62-PLC |
9 | 2015-001 | DI | PUFFMASTER2023 | 4 | SERIAL | Serial Number | 215964564-NFS |
| Expand |
|---|
| title | gt.xpt (similar to LB) |
|---|
|
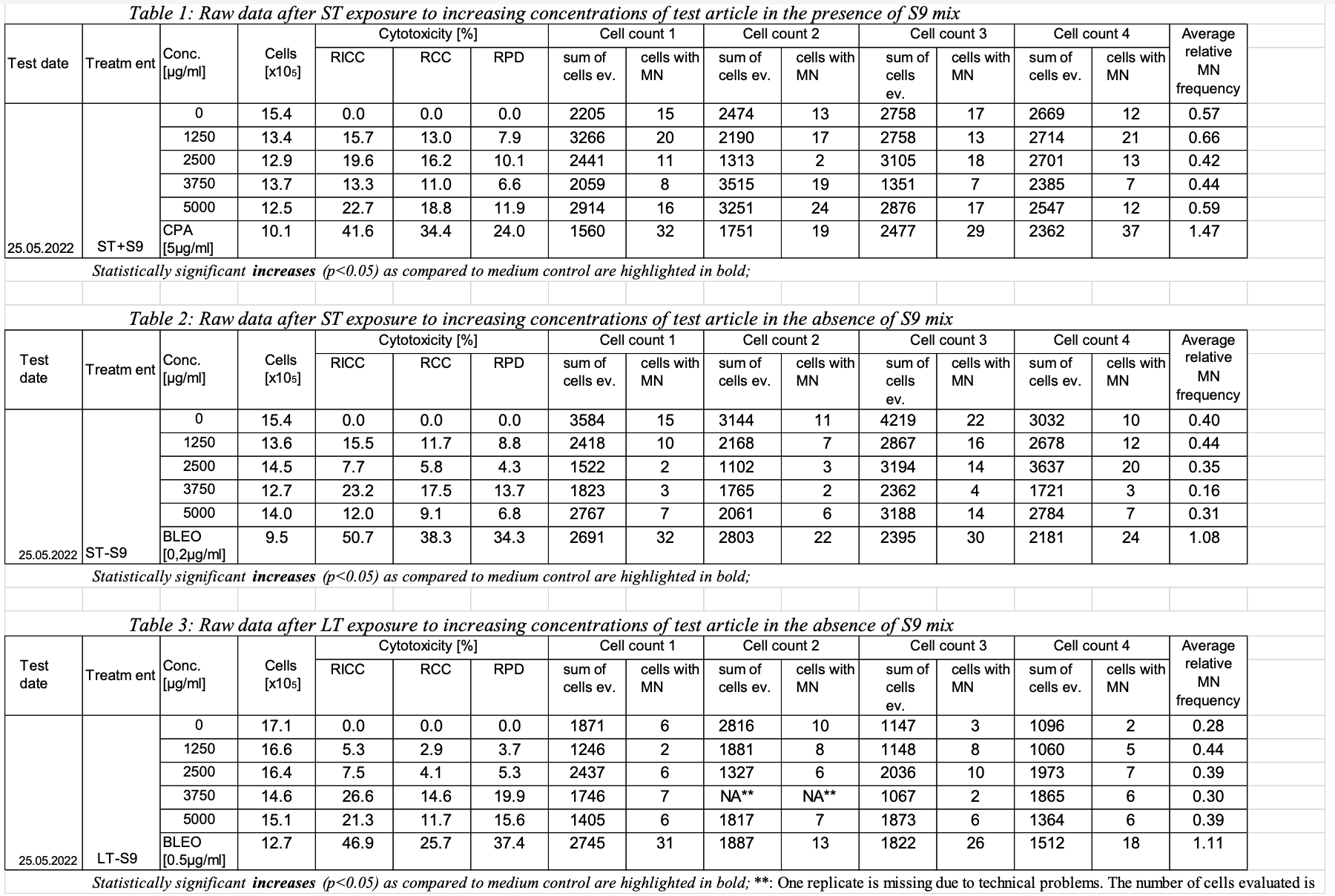
A1:  Image Removed Image Removed A2:  Image Removed Image Removed | Row | STUDYID | ASSAYID | DOMAIN | TXSETCD | GTSEQ | GTTESTCD | GTTEST | GTCELLEV
(cells evaluated) | GTORRES | GTORRESU | GTSTRESC | GTSTRESN | GTSTRESU | GTDTC |
|---|
| 1 | 123 |
|
MNvitA11| C0 | | Relative Increase in Cell Count |
|
0MNvit | A12| C0 | RCC | Relative Cell Count | 154 | 0 | % |
| 0 | 0 | % | 2022-05-25 | | 3 | 123 |
|
MNvitA13| C0 | RPD | Relative Population Doubling | 154 | 0 | % |
| 0 | 0 | % | 2022-05-25 | | 4 | 123 |
|
MNvitA14MNCELLS| MNCE | Micronucleated Cells | 2205 | 15 |
|
Cells | Cells | MNvit | A15MNCELLS| MNCE | Micronucleated Cells | 2474 | 13 |
|
Cells | Cells | MNvit | A16MNCELLS| MNCE | Micronucleated Cells | 2758 | 17 |
|
Cells | CellsMNvitA17MNCELLS| MNCE | Micronucleated Cells | 2669 |
|
12 | CellsCellsMNvitA18AVGREL | Average Relative MN Frequency| MNCECE | Micronucleated Cells/Total Cells |
| 0.57 | % |
| 0.57 | 0.57 | % | 2022-05-25 | | 9 | 123 |
|
MNvit | A21| C1250 | RICC | Relative Increase in Cell Count | 134 | 15.7 | % |
| 15.7 | 15.7 | % | 2022-05-25 | | 10 | 123 |
|
MNvit | A22| C1250 | RCC | Relative Cell Count | 134 | 13.0 | % |
| 13.0 | 13.0 | % | 2022-05-25 | | 11 | 123 |
|
MNvitA23| C1250 | RPD | Relative Population Doubling | 134 | 7.9 | % |
| 7.9 | 7.9 | % | 2022-05-25 | | 12 | 123 |
|
MNvitA24MNCELLS| MNCE | Micronucleated Cells | 3266 | 20 |
|
Cells | CellsMNvitA25MNCELLS| MNCE | Micronucleated Cells | 2190 | 17 |
|
CellsCellsMNvit | A26MNCELLS| MNCE | Micronucleated Cells | 2758 | 13 |
|
Cells | CellsMNvitA27MNCELLS| MNCE | Micronucleated Cells | 2714 | 21 |
|
Cells | CellsMNvitA28AVGREL | | MNCECE | Micronucleated Cells/Total Cells |
|
Average Relative MN Frequency...
|---|