Page History
| Request | Notes | Status | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Update Examples from TB-TAUG. | Remodel this assay and data in IS. Fix modeling mistakes in the TB TAUG Also requested by Joleen White |
| ||||||||||||||
| See his feedback in the comment section. 2023-09-07:
|
| ||||||||||||||
|
|
| Warning | ||||||||
|---|---|---|---|---|---|---|---|---|
| ||||||||
When this example is moved to the IG, make Make sure to update IS assumption #6: Measurements of cytokines, chemokines, and complement proteins should be represented in the Laboratory Test Results Results (LB) domain. Need to explain We need to draft a language to describe when to use IS vs MB vs LB for cytokine tests. See notes #3 below
|
We recently received this request: SARS CoV-2 stimulated gamma interferon [Presence] in Blood (https://loinc.org/95971-8/ --- SARS CoV-2 stimulated gamma interferon [Presence] in Blood). We also received requests for the following LOINC codes: 95974-2, 95972-6, and 95973-4. Upon further research on all 4 LOINC codes, they are referring to the IFNg Response AssayIFN-y Release Assay (IGRA), which has been modeled in the LB domain in the both TB-TAUG.TAUGs V1 and V2.
The IFNG Response test toward M. Tuberculosis is modeled in the LB domain, in the TB-TAUG v2.0 as modeling looks like the following :-
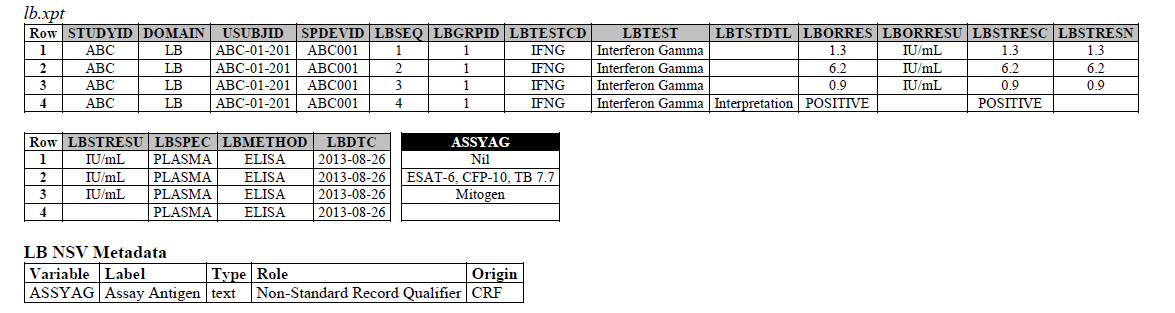
ELISA:
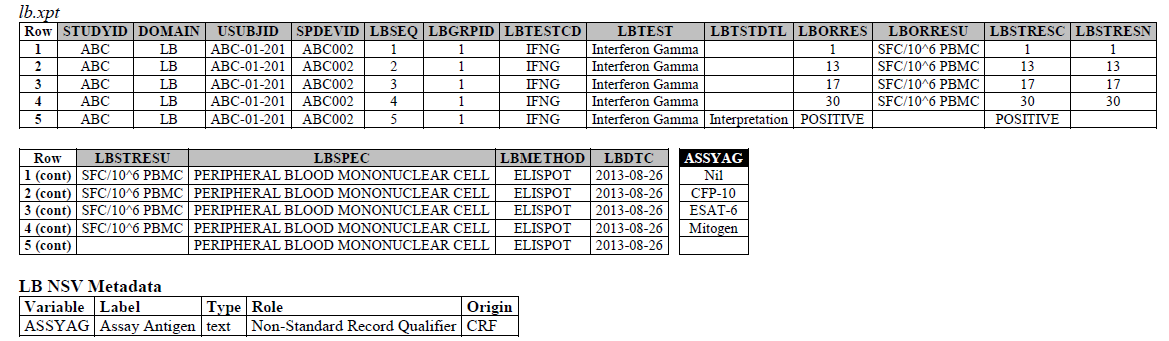
ELISPOT:
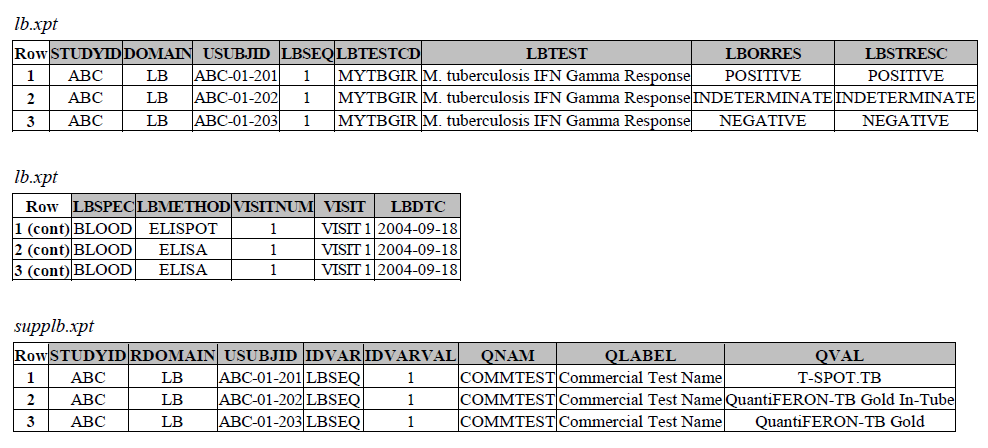
TB-TAUG v1.0 modeling looks like the following -
There are several concerns with this modeling:
- There are two ways of modeling Interferon Gamma Release Assay based on TB-TAUG V1 and V2, does V2 modeling replace V1 modeling? Are they modeling the same thing?
- Email sent to Jon to see if V2 model is intended to replace V1 model.
- Email from Jon 2022-07-07: I was indeed part of the team but have no recollection of this example or any of the discussions around how it evolved. However, I think it’s safest to go with TBv2; it must have been updated for a reason. I would deprecate the original TESTCD, especially if you’ve already been denying additional requests for tests following that same structure.
- Where do you show that this challenge assay is done against M. Tuberculosis (or any microbe of interest, like SARS-CoV-2), where do you map M. TbTuberculosis? - NHOID is NOT appropriate and should NOT be used here because in this case, the subject of study is NOT the Mycobacterium tuberculosis itself but whether someone has been previously exposed to M. Tuberculosis, by examining whether there is heightened Interferon-gamma activation. A positive result does not mean the person is currently infected with Mycobacterium tuberculosis, it may mean that the person had been previously infected by, or vaccinated against M. Tuberculosis. NHOID is used when you know the microorganism or a reference strain is present in the testing sample. M. Tuberculosis is mapped to ISCNDAGT to indicate that the stimulating agents are TB antigens.
Info title When should NHOID be used? NHOID, defined by the Non-host Organism Identifiers (OI) domain, should be used to map microorganisms that have been either experimentally determined in the course of a study or are previously known (e.g., lab strains used as reference in the study). In other words, NHOID is used when the study subject is the microorganism, and when the microorganism is present in the testing sample. In vaccine efficacy studies, a subject’s post-immunization sera is often incubated with a microbial strain of interest, where the functional capacities of the vaccine-induced antibodies are measured through whether the antibodies can effectively stop (from infection), neutralize, and kill the study microorganism of interest, in vitro. Examples of such tests include microneutralization, hemagglutination inhibition, and opsonophagocytic-killing assays. These are tests which measure the direct effect of the antimicrobial antibodies on the microorganism; therefore, said microorganism is the study subject and should be mapped to NHOID.
Tests that measure and quantify a subject's cellular and humoral immune responses to a microorganism or a vaccination agent—such as measurements of activated cytokine- or antibody–secreting cells, or cytokine response assays (e.g., interferon gamma response test)—are biological measurements about the human subject. Because these are not assessments about the microorganism itself, NHOID should not be used.
Status colour Green title Team agrees - Lastly, Interferon-Gamma Response Assay is a classic immunological test, is it correct to map this to the LB domain?
- Can we model this in the IS domain, see example dataset below. Yes -
Status colour Green title Team Agrees - If we model this test in IS, how do we distinguish it from the INFg test in lab. If we are running a routine lab test looking for IFNg levels it goes to LB. If we are running IFNg challenge/response test toward a specific, known microorganism then it goes to IS, you will also need to use the ISCNDAGT variable to house the challenge agent name. Yes-
Status colour Green title Team Agrees
- Can we model this in the IS domain, see example dataset below. Yes -
...
- Map to NHOID.
- Map to NHOID.
- Interferon-Gamma Release Assay (IGRA) is a classic immunological test, is it correct to map this to the LB domain as per the TB TAUGs? What recommendations do we give to our users in the future when we see a request such as: SARS-CoV-2 stimulated gamma interferon, https://loinc.org/95971-8/?
- Model in IS, if you need to report the detailed changes in the actual levels of IFN-y or number of IFN-y secreting cells, the stimulating and control agents used in the assay, and a "summary interpretation" result of positive, negative, or indeterminate, etc. In this case, you are testing and recording the explicit changes in the subject/host's IFN-y release immune response, and this belongs in the IS domain. A derived MB record may be created to show that M. Tuberculosis is identified/detected based on the interpretation of the IS dataset.
- or...
- Model and report in MB ONLY, if you do NOT care or the lab does not provide you with actual details and results of the subject/host's IFN-y levels related changes. In this case, you are doing a microbial detection/identification test based on the interpretation (MBTSTDTL) of the INTERFERON GAMMA RELEASE ASSAY (MBMETHOD), see here: https://loinc.org/71773-6/.
- The ELISPOT LB example in the TB-TAUG V2 has an error, the unit is reported in SFC/10^6 PBMC so you are counting the number of cells that secrete
...
- IFN-y. The example had a LBTEST
...
- = interferon gamma, this is wrong,
...
- the test should be measured in Cells, and should be changed to ISTEST = Cytokine-secreting Cells, where ISMSCBCE = Interferon Gamma.
TB-TAUG v2.0 Remodel in the IS domain
Model in IS, if you need to report changes in the actual level of IFN-y or number of IFN-y secreting cells, the stimulating and control agents used in the assay, and a "summary interpretation" result of positive, negative, or indeterminate, etc. In this case, you are testing and recording the explicit changes in the IFN-y release immune response, and this belong in the IS domain.
Example 1: Measure INF-y using T-Cell ELISPOT
If we were to model this in IS:
T-Cell ELISPOT:
| Dataset wrap | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
MBTEST = M. tuberculosis IFN Gamma Response - OK to deprecate
Note: in the future when we see this type of requests, recommend people to either model in IS or MB, but this is an "or" situation where you do not expect to see both IS and MB. It is one or the other.
Example 2: Measure INF-y using ELISA
Jordan Li When moving this example to the Example Collection for internal and public review, update the example description to mention that: ISCNDAGT is NOT controlled so this could be ESAT-6, CFP-10 or other peptide (instead of TB1 and TB2 Antigens). We are not suggesting that the stimulating agents used for TB IFN-y test have to be TB1/2 Antigens. This is just an example to show which variable needs to be used to populate which type of data, in order to make a meaningful and unique record. It is up to the sponsor to decide what collected value should be mapped to ISCNDAGT.
| Dataset wrap | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dataset wrap | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dataset wrap | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dataset2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Row | STUDYID | DOMAIN | SPDEVID | MBSEQ | DIPARMCD | DIPARM | DIVAL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1 | ABC | DI | ABC002 | 1 | DEVTYPE | Device Type | INTERFERON-GAMMA RELEASE ASSAY | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | ABC | DI | ABC002 | 2 | TRADENAM | Trade Name | T-Spot.TB |
ELISA:
|
Example 3: Identification of Mycobacterium tuberculosis
The MB data below are derived records from the IS dataset above, the identification and diagnosis of Mycobacterium tuberculosis are interpretations based on the IFNy responses test results above.
Note: there is an existing, pre-coordinated MBTEST = M. tuberculosis IFN Gamma Response/C92241, (also from TB TAUG V1), this test will be deprecated because the modeling is incorrect. The MB/IS team will not publish tests similar to C92241 in the future, and we recommend users to model this type of tests using the below structure. Please be aware that you may also ONLY report the MB records below alone, if you do not care or the lab does not provide you with the actual results and details of the subject's IFN-y levels related changes.
| Dataset wrap | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Dataset wrap | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
The RELREC dataset below shows the relationship between IS and MB.
| Dataset wrap | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|