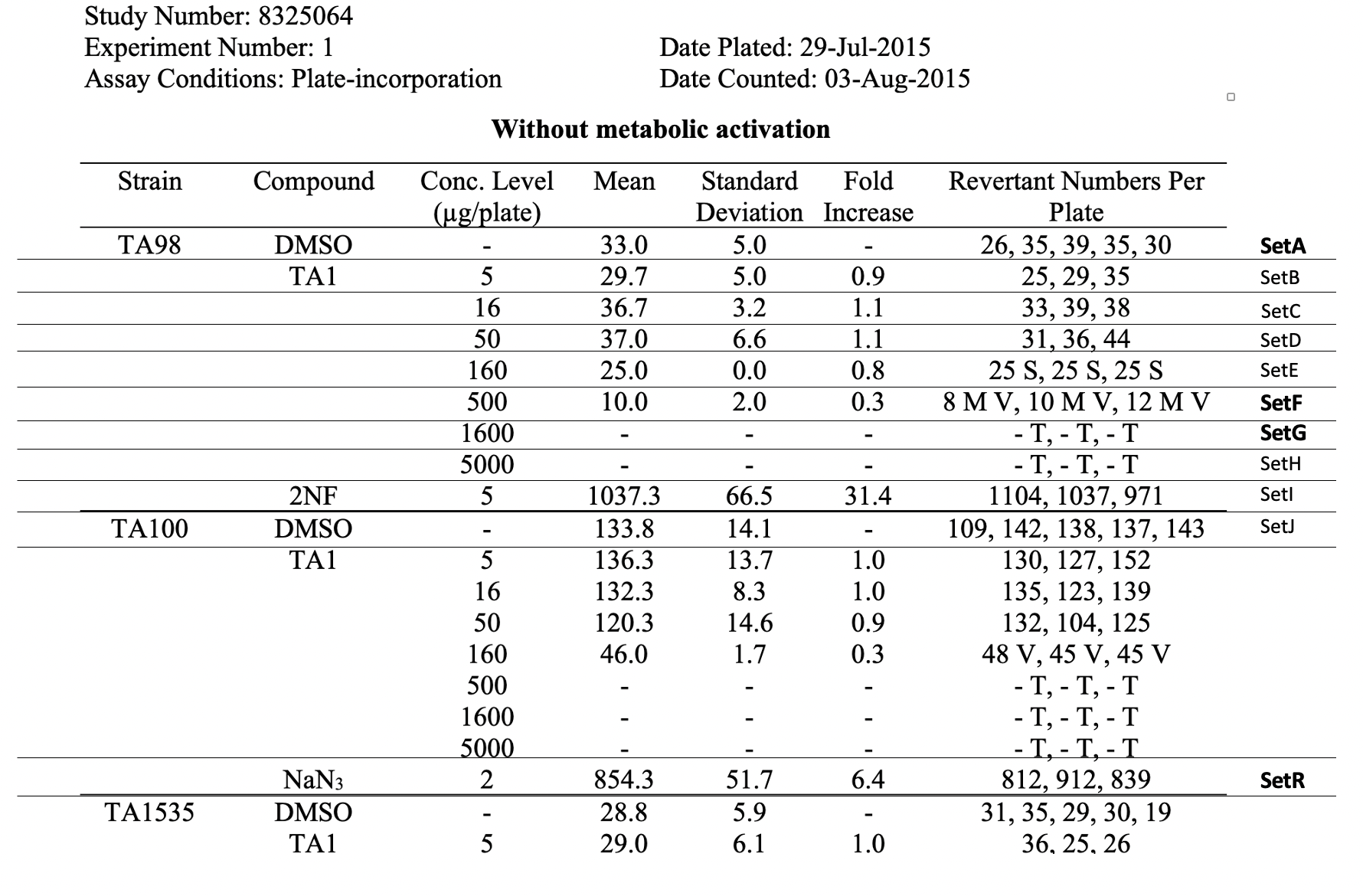
| Row | STUDYID | ASSAYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|
| 1 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 1 | SPECIES | Species | Salmonella typhimurium |
| 2 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 2 | STRAIN | Strain/Substrain | TA98 |
3 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 3 | METACT | Metabolic Activation | | 4 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 4 | METACTFL | Y/N presence of metabolic activation | N |
| 5 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 5 | TRTDMIN | Treatment Duration Minimum | 71.5 |
| 6 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 6 | TRTDTRG | Treatment Duration Target | 72 |
| 7 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 7 | TRTDMAX | Treatment Duration Maximum | 72.5 |
| 8 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 8 | TRTDU | Treatment Duration Unit | HOURS |
| 9 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 9 | INCBTMP | Incubation Temperature | 37 |
| 10 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 10 | INCBTMPU | Incubation Temperature Unit | C |
| 11 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 11 | HUMID | Atmospheric Relative Humidity Percent | 50 |
| 12 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 12 | ATMCO2 | Atmospheric CO2 Percent | 5 |
| 13 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 13 | SPTOBID | Sponsor defined tobacco identifier | CIG01a |
| 14 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 14 | EXPTYP | Exposure Type (See TIG NC workstream minutes 30-Jan here: Nonclinical) | Submerged |
| 15 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 15 | SAMTYP | Sample Type | Total Particulate Matter in PBS |
16 | 8325064 | Ames | | Row | STUDYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|
| 1 | 8325064 |
16INTRVN | name of the intervention article | TA1 | | SPECIES | Species | SALMONELLA TYPHIMURIUM |
| 2 |
17Ames | 17ITVTYPE | type of intervention article | Vehicle Control | | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 |
| 3 |
18Ames | 18ITVCONC | Concentration of intervention article | 0 | | IVTDTRG | In vitro Treatment Duration Target | 72 |
| 4 | 8325064 |
19 | 8325064 | Ames19ITVCONCU | Concentration Unit | ug/plate | | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 |
| 5 |
20Ames | 20TRTV VehicleDMSO21Ames | 21SPDEVID | Sponsor defined device identifier | PUFFMASTER3K | | INCBTMP | Incubation Temperature | 37 |
| 7 |
22Ames | 22DUREFID | Smoke Regimen | Medium Intensity Regimen | | INCBTMPU | Incubation Temperature Unit | C |
| 8 |
23Ames | SetFFC5001SPECIES | Species | Salmonella typhimurium | | ATMRHP | Atmospheric Relative Humidity Percent | 50 |
| 9 |
24Ames | SetFFC5002STRAIN | Strain/Substrain | TA98 | | ATMCO2P | Atmospheric CO2 Percent | 5 |
| 10 | 8325064 |
25 | 8325064 | AmesSetFFC5003 | METACT | Metabolic Activation | | SPTOBID | Applicant-defined tobacco identifier | CIG01a |
| 11 |
26Ames | SetFFC5004METACTFL | Y/N presence of metabolic activation | N | 27Ames | SetFFC5005TRTDMIN | Treatment Duration Minimum | 71.5 | | SAMTYP | Sample Type | Total Particulate Matter in PBS |
| 13 | 8325064 |
28 | 8325064 | AmesSetFFC5006TRTDTRG | Treatment Duration Target | 72 | | APDEVID | Applicant-defined device identifier | PUFFMASTER3K |
| 14 |
29Ames | SetFFC5007TRTDMAX | Treatment Duration Maximum | 72.5 | | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN |
| 15 |
30Ames | SetFFC5008TRTDU | Treatment Duration Unit | HOURS | | STRAIN | Strain/Substrain | TA98 |
| 16 | 8325064 |
31 | 8325064 | AmesSetFFC5009INCBTMP | Incubation Temperature | 37 | | MTACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
| 17 |
32Ames | SetFFC50010INCBTMPU | Incubation Temperature Unit | C | | METACTFL | Presence of Metabolic Activation Flag | N |
| 18 |
33Ames | SetFFC50011HUMID | Atmospheric Relative Humidity Percent | 50 | | ITVNAM | Intervention Article Name | DMSO |
| 19 |
34Ames | SetFFC50012ATMCO2 | Atmospheric CO2 Percent | 5 | 35 | 8325064 | | ITVTYPE | Intervention Article Type | VEHICLE |
| 20 | 8325064 |
AmesSetFFC50013SPTOBID | Sponsor defined tobacco identifier | CIG01a | | ITVCONC | Intervention Article Concentration | 100 |
| 21 |
36Ames | SetFFC50014| 37 | 8325064 | Ames | TX | SetF | F-TA98-C500 | 15 | SAMTYP | Sample Type | Total Particulate Matter in PBS |
38 | 8325064 | Ames | EXPTYP | Exposure Type (See TIG NC workstream minutes 30-Jan here: Nonclinical) | Submerged | | ITVCONCU | Intervention Article Concentration Unit | % |
| 22 | 8325064 | TX | SetA | A-TA98-C0 | 22 | TRTV | Treatment Vehicle | DMSO |
| 23 | 8325064 |
16INTRVN | name of the intervention article | TA1 | | SPECIES | Species | SALMONELLA TYPHIMURIUM |
| 24 | 8325064 |
39 | 8325064 | Ames17ITVTYPE | type of intervention article | Vehicle Control | | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 |
| 25 | 8325064 |
40 | 8325064 | Ames18ITVCONC | Concentration of intervention article | 0 | | IVTDTRG | In vitro Treatment Duration Target | 72 |
| 26 |
41Ames | 19ITVCONCU | Concentration Unit | ug/plate | | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 |
| 27 |
42Ames | 20TRTV VehicleDMSO43Ames | 21SPDEVID | Sponsor defined device identifier | PUFFMASTER3K | | INCBTMP | Incubation Temperature | 37 |
| 29 |
44Ames | 22DUREFID | Smoke Regimen | Medium Intensity Regimen | 45 | 46 | 47 | 48 | 8325064 | Ames | TX | SetG (row 7) do G not I!!! | METACT | Metabolic Activation (should there be two parms? Presence, type)? | None | 8325064 | Ames | TX | SetG | TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) | 8325064 | Ames | TX | SetG | TRTDRTOL | Treatment Duration Tolerance | 8325064 | Ames | TX | SetG | TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | 8325064 | Ames | TX | SetG | INTRVN | name of the intervention article (Tobacco ProdA, Bleomycin or Cyclophosphamid A) | 2-Nitrofluorine | 8325064 | Ames | TX | SetG | ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Positive Control | 8325064 | Ames | TX | SetG | ITVCONC | Concentration of intervention article | 5 | 8325064 | Ames | TX | SetG | ITVCONCU | Concentration Unit | ug/plate | 8325064 | Ames | TX | SetG | REGIME | Smoking Regime | ISO | ... | SetG | 8325064 | Ames | TX | SetR (Row 18) | METACT | Metabolic Activation (should there be two parms? Presence, type)? | None | 8325064 | Ames | TX | SetR | TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) | 8325064 | Ames | TX | SetR | TRTDRTOL | Treatment Duration Tolerance | 8325064 | Ames | TX | SetR | TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | 8325064 | Ames | TX | SetR | INTRVN | name of the intervention article (Tobacco ProdA, Bleomycin or Cyclophosphamid A) | 4-Nitroquinoline-1-oxide | 8325064 | Ames | TX | SetR | ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Positive Control | 8325064 | Ames | TX | SetR | ITVCONC | Concentration of intervention article | 2 | 8325064 | Ames | TX | SetR | ITVCONCU | Concentration Unit | ug/plate | 8325064 | Ames | TX | SetR | STRAIN | Strain/Substrain | TA98 | 8325064 | Ames | TX | SetR | REGIME | Smoking Regime | ISO |