| Panel |
|---|
1: Discuss the PCTEST and PCTESTCD Controlled Terminology. They are always measuring nicotine. |
This is an example of a study designed to evaluate plasma nicotine pharmacokinetic (PK) parameters following the use of nicotine pouches. Three Two different nicotine pouches products were used in the study. Each pouch product was evaluated in a 180-minute test session . AUC and CMAX were to be determined. The study consisted of a screening period, one day of admission, four separate days of on-site product use with 1–3 with 1-3 days in between each product use, and a 7-day safety follow-up period. Plasma samples were taken at 45, 30, and 15 minutes prior to the start of product use and 2, 4, 7, 10, 15, 20, 30, 40, 50, 60, and 120 , and 240 minutes after the start of product use. (Cmax), time to reach Cmax (tmax), and the baseline-corrected area under the plasma concentration–time curves (i) from start of product use (t0) to the last quantifiable nicotine concentration time point (AUC0-last) and (ii) from t0 to 10 minutes after t0 (AUC0-10’). The pharmacokinetic parameters were derived from plasma nicotine concentrations-versus-time data by means of non-compartmental analysis using Phoenix WinNonlin (version 6.2, Pharsight Corp, Sunnyvale, California) and corrected for baseline following the method described by Kraiczi et al.30 whereby the baseline (C0) was defined as the average concentration of the three time points prior to t0 (45, 30, and 15 minutes prior to t0) of each visit from whose slope the elimination rate constant (k) was estimated and then used for the calculation of the baseline corrected values.
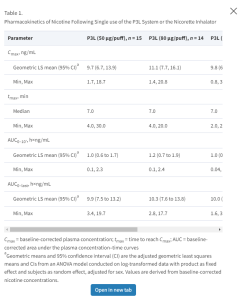 Image Removed
Image Removed
More time points are often used; these timepoints presented were used only for illustration to save space.)
The PCLLOQ for the analytes measured were reported; PCULOQs were not reported.
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Rows 1-23: | Show the day 1 pre-dose drug and metabolite concentrations of nicotine in plasma and urine. | | Rows 3-4: | Show day 1 pre-dose drug and metabolite concentrations in urine. Urine specimens may be collected over an interval; both PCDTC and PCENDTC have been populated with the same value to indicate that these specimens were collected at a point in time rather than over an interval. | | Rows 5-6: | Show specimen properties (VOLUME and PH) for the day 1 pre-dose urine specimens. These have a PCCAT value of "SPECIMEN PROPERTY". | | Rows 7-12: | Show day 1 post-dose drug and metabolite concentrations in plasma. | | Rows 13-16: | Show day 11 drug and metabolite concentrations in plasma. | | Rows 17-20: | Show day 11 drug and metabolite concentrations in urine specimens collected over an interval. The elapsed times for urine samples are calculated as the elapsed time (from the reference time point, PCTPTREF) to the end of the specimen collection interval. Elapsed time values that are the same for urine and plasma samples have been assigned the same value for PCTPT. For the urine samples, the value in PCEVLINT describes the planned evaluation (or collection) interval relative to the time point. The actual evaluation interval can be determined by subtracting PCDTC from PCENDTC. | | at 45, 30 and 15 min before start of delivery of the nicotine. PCDTC is populated to indicate when these specimens were collected. | | Rows 4-8: | Show the day 1 concentrations of nicotine in plasma after the start of delivery of the nicotine. | | Rows 9-16: | Show the day 4 pre- and post-dose concentrations of nicotine in plasma. | | Rows 17-18: | Show the day 1 pre-dose plasma concentrations of cotinine and N'-Nitrosonornicotine in plasma | Rows 21-30: | Show additional drug and metabolite concentrations and specimen properties related to the day 11 dose. |
|
| Dataset2 |
|---|
| Row | STUDYID | DOMAIN | USUBJID | PCSEQ | PCGRPID | PCREFID | PCTESTCD | PCTEST | PCCAT | PCSPEC | PCORRES | PCORRESU | PCSTRESC | PCSTRESN | PCSTRESU | PCSTAT | PCLLOQPCULOQ | VISITNUM | VISIT | VISITDY | PCDTC | PCENDTC | PCDY | PCTPT | PCTPTNUM | PCTPTREF | PCRFTDTC | PCELTM | PCEVLINT |
|---|
| 1 | ABC-123A123 | PC | 123-0001A2008 | 1 | Day 1 | A554134-10 | NICO_PAR | A10 | NICOTNE | NicotineNICOTINE PARENT | ANALYTE | PLASMA | <0.1 | ng/mL | <0.1 |
| ng/mL | 0.10 | 20 | 1 | DAY 1 | 1 | 2001-02-01T07:4515 | 1 | 45MIN 45 MIN PREDOSE | 01 | Day 1 Dose | 20012021-02-01T08:00 | -PT45M | NICO_PAR | NICOTINE PARENT PLASMA | <1.0 | | 30 MIN PREDOSE | -PT30M | 2 | ABC-123A123 | PC | 123-0001A2008 | 2 | Day 21 | A554134-10 | DRGA_PAR | A11 | NICOTINE | Drug A Parent | ANALYTE | PLASMA | <1.0 | ng/mL | <0<1.10 | ng/mL |
|
| 0.1020 | 1DAY 1 | Day1 | 1 | 2001-02-01T07:4530 | 1 | 15 MIM 30 MIN PREDOSE | 02 | Day 1 Dose | 20012021-02-01T08:00 | -PT15MPT30M | | 3 | ABC-123A123 | PC | 123-0001A2008 | 3 | Day 3 | A554134-11 | DRGA_MET | Day 1 | A12 | NICOTINE | NicotineDrug A Metabolite | ANALYTE | PLASMA | 1<1.50 | ng/mL | <2<0.1 |
| ng/mL | 20.0010 | 500 | 1 | DAY 1 | 1 | 2001-02-01T07:45 | 2001-02-01T07:45 | 12M | 15 MIN PREDOSE | 03 | Day 1 Dose | 20012021-02-01T08:00 | PT2M-PT15M | | 4 | ABC-123A123 | PC | 123-0001A2008 | 4 | Day 1 | A554134-11 | DRGA_PAR | A13 | NICOTINE | NicotineDrug A Parent | ANALYTE | PLASMA | 31.05 | ng/mL | 1.5 | 1.5<2 | ng/mL | 20.0010 | 500 | 1 | DAY 1 | 1 | 2001-02-01T0701T08:452001-02-01T07:45 | 14M | 2 MIN | 04 | Day 1 Dose | 20012021-02-01T08:00 | PT$MPT2M | | 5 | ABC-123A123 | PC | 123-0001A2008 | 5 | Day 1 | A554134-11A14 | VOLUMENICOTINE | VolumeNicotine | SPECIMEN PROPERTYANALYTE | PLASMA | 83.0 | ng/mL | 100 | 100 | mL | 3.0 | 3.0 | ng/mL | 0.10 | 1 | DAY 1 | 1 | 2001-02-01T07:452001-02-01T07:4501T08:04 | 18M | 4 MIN | 05 | Day 1 Dose | 20012021-02-01T08:00 | PT8MPT4M | | 6 | ABC-123A123 | PC | 123-0001A2008 | 6 | Day 1 | A554134-11A15 | PHNICOTINEPH | Nicotine | SPECIMEN PROPERTYANALYTE | PLASMA | 58.0 | ng/mL | 8.0 | 5.5 | 8.0 | ng/mL | 0.105.5 | 1 | DAY 1 | 1 | 2001-02-01T0701T08:45 | 2001-02-01T07:45 | 08 | 160M | 8 MIN | 06 | Day 1 Dose | 20012021-02-01T08:00 | PT60PT8M | | 7 | ABC-123A123 | PC | 123-0001A2008 | 7 | Day 1 | A554134-12 | DRGA_MET | A16 | NICOTINE | NicotineDrug A Metabolite | ANALYTE | PLASMA | 35.0. | ng/mL | 5.40 | 5.40 | ng/mL | 0.10 | 20 | 1 | DAY 1 | 1 | 2001-02-01T09:3000 | 1 | 120M | 60 MIN | 71.5 | Day 1 Dose | 20012021-02-01T08:00 | PT120PT60 | | 8 | ABC-123A123 | PC | 123-0001A2008 | 8 | Day 1 | A554134-12 | DRGA_PAR | A16 | NICOTINE | NicotineDrug A Parent | ANALYTE | PLASMA | 23.0 0 | ng/mL | 43.7404 | 3.740 | ng/mL | 0.10 | 20 | 1 | DAY 1 | 1 | 2001-02-01T0901T010:3000 | 1 | 240M | 120 MIN | 81.5 | Day 1 Dose | 20012021-02-01T08:00 | PT240MPT120 | | 9 | ABC-123A123 | PC | 123-0001A2008 | 9 | Day 14 | A554134-13 | DRGA_MET | A17 | NICOTINE | NicotineDrug A Metabolite | ANALYTE | PLASMA | 5.44 | ng/mL | 5.44 | 5.44 | ng/mL | 0.10 | 202 | 1 | DAY 141 | 4 | 2001-02-01T1404T107:0015 | 146H | 45 MIN PREDOSE | 61 | Day 1 4 Dose | 20012021-02-01T0804T08:00 | PT6H00M-PT45M | | 10 | ABC-123A123 | PC | 123-0001A2008 | 10 | Day 14 | A554134-13 | DRGA_PAR | A18 | NICOTINE | NicotineDrug A Parent | ANALYTE | PLASMA | 1.09 | ng/mL | 1.09 | 1.09 | ng/mL | 0.10 | 20 | 12 | DAY 141 | 4 | 2001-02-01T1404T07:0030 | 146H | 30 MIN PREDOSE | 62 | Day 1 4 Dose | 20012021-02-01T0804T08:00 | PT6H-PT30M | | 11 | ABC-123A123 | PC | 123-0001A2008 | 11 | Day 14 | A554134-14 | DRGA_MET | A19 | NICOTINE | NicotineDrug A Metabolite | ANALYTE | PLASMA | NOT DONE | 20 | 2 | DAY 2 | 2 | 2001-02-02T08:00 | 2 | 24H | 24 | Day 1 Dose | 2001-02-01T08:00 | PT24H | 12 | ABC-123 | PC | 123-0001 | 12 | Day 1 | A554134-14 | DRGA_PAR | Drug A Parent | ANALYTE | PLASMA | <0.1 | ng/mL | <0.1 |
| ng/mL | 0.1020 | 2 | DAY 242 | 4 | 2001-02-02T0804T07:0015 | 2424H | 15 MIN REDOSE | 243 | Day 1 4 Dose | 20012021-02-01T0804T08:00 | PT24H-PT15M | | 13 | ABC-123A123 | PC | 123-0001A2008 | 1312 | Day 114 | A554134-15 | DRGA_MET | A20 | NICOTINE | NicotineDrug A Metabolite | ANALYTE | PLASMA | 3.41 | ng/mL | 3.41 | 3.41 | ng/mL | 0.10 | 202 | 3 | DAY 11411 | 4 | 2001-02-11T07:45 | 11 | PREDOSE | 0 | Day 11 Dose | 04T08.02 | 4 | 2 MIN | 4 | Day 4 Dose | 20212001-02-11T0804T08:00-PT15M | PT2M | | 1413 | ABC-123A123 | PC | 123-0001A2008 | 1413 | Day 114 | A554134-15 | DRGA_PAR | A21 | NICOTINE | NicotineDrug A Parent | ANALYTE | PLASMA | <0.1 | ng/mL | <0.1 |
| ng/mL | 0.10 | 202 | 3 | DAY 11411 | 4 | 2001-02-11T0704T08:4504 | 114 | PREDOSE4 MIN | 05 | Day 11 4 Dose | 20012021-02-11T0804T08:00-PT15M | PT4M | | 1514 | ABC-123A123 | PC | 123-0001A2008 | 1514 | Day 114 | A554134-16 | DRGA_MET | A22 | NICOTINE | NicotineDrug A Metabolite | ANALYTE | PLASMA | 8.74 | ng/mL | 8.74 | 8.74 | ng/mL | 0.10 | 202 | 3 | DAY 11411 | 4 | 2001-02-11T0904T008.:3008 | 114 | 1H30MIN | 8 MIN | 61.5 | Day 11 4 Dose | 20012021-02-11T0804T08:00 | PT1H30MPT8M | | 1615 | ABC-123A123 | PC | 123-0001A2008 | 1615 | Day 114 | A554134-16 | DRGA_PAR | A23 | NICOTINE | NicotineDrug A Parent | ANALYTE | PLASMA | 4.2 | ng/mL | 4.2 | 4.2 | ng/mL | 0.10 | 20 | 32 | DAY 11411 | 4 | 2001-02-11T0904T09:3000 | 114 | 1H30MIN | 60 MIN | 71.5 | Day 11 4 Dose | 20012021-0204-11T08:00 | PT1H30MPT160M | | 1716 | ABC-123A123 | PC | 123-0001A2008 | 1716 | Day 114 | A554134-17 | DRGA_MET | A24 | NICOTINE | NicotineDrug A Metabolite | ANALYTE | URINEPLASMA | 245 | ng/mL | 245 | 245 | ng/mL | 2.00 | 500 | 3 | DAY 11 | 11 | 2001-02-11T08:00 | 2001-02-11T14:03 | 11 | 6H | 6 | Day 11 Dose | 2001-02-11T08:00 | PT6H | -PT6H | 18 | ABC-123 | PC | 123-0001 | 18 | Day 11 | A554134-17 | DRGA_PAR | Drug A Parent | ANALYTE | URINE | 13.1 | ng/mL | 13.1 | 13.1 | ng/mL | 2.00 | 500 | 3 | DAY 11 | 0.10 | 2 | DAY 4 | 411 | 2001-02-11T0804T010:00 | 2001-02-11T14:03 | 11 | 6H | 6 | Day 11 Dose | 4 | 120 MIN | 8 | Day 4 Dose | 2021-042001-02-11T08:00 | PT6H | -PT6H | 19 | ABC-123 | PC | 123-0001 | 19 | Day 11 | A554134-17 | VOLUME | Volume | SPECIMEN PROPERTY | URINE | 574 | mL | 574 | 574 | mL | 3 | DAY 11 | 11 | 2001-02-11T08:00 | 2001-02-11T14:03 | 11 | 6H | 6 | Day 11 Dose | 2001-02-11T08:00 | PT6H | -PT6H | 20 | ABC-123 | PC | 123-0001 | 20 | Day 11 | A554134-17 | PH | PH | SPECIMEN PROPERTY | URINE | 5.5 | 5.5 | PT120M | | 17 | A123 | PC | A2008 | 17 | Day 1 | A10 | COTININE | Cotinine | ANALYTE | PLASMA | 1 | 5.5 | 3 | DAY 11 | 11 | 2001-02-11T08:00 | 2001-02-11T14:03 | 11 | 6H | 6 | Day 11 Dose | 2001-02-11T08:00 | PT6H | -PT6H | 21 | ABC-123 | PC | 123-0001 | 21 | Day 11 | A554134-18 | DRGA_MET | Drug A Metabolite | ANALYTE | PLASMA | 9.02 | ng/mL | 9.02 | 9.02 | ng/mL | 0.10 | 20 | 3 | DAY 11 | 11 | 2001-02-11T14:00 | 11 | 6H | 6 | Day 11 Dose | 2001-02-11T08:00 | PT6H | 22 | ABC-123 | PC | 123-0001 | 22 | Day 11 | A554134-18 | DRGA_PAR | Drug A Parent | ANALYTE | PLASMA | 1.18 | ng/mL | 1.18 | 1.18 | ng/mL | 0.10075 | 20 | 31 | DAY 11111 | 1 | 2001-02-11T14:00 | 11 | 6H | 6 | Day 11 Dose | 01T07:15 | 1 | 45 MIN PREDOSE | 1 | Day 1 Dose | 2021-02-01T08:00 | -PT45M | | 18 | A123 | PC | A2008 | 18 | Day 1 | A10 | NNN | N'-Nitrosonornicotine | ANALYTE | PLASMA | <0.1 | pg/mL | <.3 |
| pg/mL | 0.3 | 1 | DAY 1 | 1 | 2001-02-01T07:15 | 1 | 45 MIN PREDOSE | 1 | Day 1 Dose | 2021-02-01T08:00 | -PT45M | 2001-02-11T08:00 | PT6H | 23 | ABC-123 | PC | 123-0001 | 23 | Day 11 | A554134-19 | DRGA_MET | Drug A Metabolite | ANALYTE | URINE | 293 | ng/mL | 293 | 293 | ng/mL | 2.00 | 3 | DAY 11 | 11 | 2001-02-11T14:03 | 2001-02-11T20:10 | 11 | 12H | 12 | Day 11 Dose | 2001-02-11T08:00 | PT12H | -PT6H | 24 | ABC-123 | PC | 123-0001 | 24 | Day 11 | A554134-19 | DRGA_PAR | Drug A Parent | ANALYTE | URINE | 7.1 | ng/mL | 7.1 | 7.1 | ng/mL | 2.00 | 3 | DAY 11 | 11 | 2001-02-11T14:03 | 2001-02-11T20:10 | 11 | 12H | 12 | Day 11 Dose | 2001-02-11T08:00 | PT12H | -PT6H | 25 | ABC-123 | PC | 123-0001 | 25 | Day 11 | A554134-19 | VOLUME | Volume | SPECIMEN PROPERTY | URINE | 363 | mL | 363 | 363 | mL | 3 | DAY 11 | 11 | 2001-02-11T14:03 | 2001-02-11T20:10 | 11 | 12H | 12 | Day 11 Dose | 2001-02-11T08:00 | PT12H | -PT6H | 26 | ABC-123 | PC | 123-0001 | 26 | Day 11 | A554134-19 | PH | PH | SPECIMEN PROPERTY | URINE | 5.5 | 5.5 | 5.5 | 3 | DAY 11 | 11 | 2001-02-11T14:03 | 2001-02-11T20:10 | 11 | 12H | 12 | Day 11 Dose | 2001-02-11T08:00 | PT12H | -PT6H | 27 | ABC-123 | PC | 123-0001 | 27 | Day 11 | A554134-20 | DRGA_MET | Drug A Metabolite | ANALYTE | URINE | 280 | ng/mL | 280 | 280 | ng/mL | 2.00 | 4 | DAY 12 | 12 | 2001-02-11T20:03 | 2001-02-12T08:10 | 12 | 24H | 24 | Day 11 Dose | 2001-02-11T08:00 | PT24H | -PT12H | 28 | ABC-123 | PC | 123-0001 | 28 | Day 11 | A554134-20 | DRGA_PAR | Drug A Parent | ANALYTE | URINE | 2.4 | ng/mL | 2.4 | 2.4 | ng/mL | 2.00 | 4 | DAY 12 | 12 | 2001-02-11T20:03 | 2001-02-12T08:10 | 12 | 24H | 24 | Day 11 Dose | 2001-02-11T08:00 | PT24H | -PT12H | 29 | ABC-123 | PC | 123-0001 | 29 | Day 11 | A554134-20 | VOLUME | Volume | SPECIMEN PROPERTY | URINE | 606 | mL | 606 | 606 | mL | 4 | DAY 12 | 12 | 2001-02-11T20:03 | 2001-02-12T08:10 | 12 | 24H | 24 | Day 11 Dose | 2001-02-11T08:00 | PT24H | -PT12H | 30 | ABC-123 | PC | 123-0001 | 30 | Day 11 | A554134-20 | PH | PH | SPECIMEN PROPERTY | URINE | 5.5 | 5.5 | 5.5 | 4 | DAY 12 | 12 | 2001-02-11T20:03 | 2001-02-12T08:10 | 12 | 24H | 24 | Day 11 Dose | 2001-02-11T08:00 | PT24H | -PT12H | 31 | ABC-123 | PC | 123-0001 | 31 | Day 11 | A554134-21 | DRGA_MET | Drug A Metabolite | ANALYTE | PLASMA | 3.73 | ng/mL | 3.73 | 3.73 | ng/mL | 0.10 | 20 | 4 | DAY 12 | 12 | 2001-02-12T08:00 | 12 | 24H | 24 | Day 11 Dose | 2001-02-11T08:00 | PT24H | 32 | ABC-123 | PC | 123-0001 | 32 | Day 11 | A554134-21 | DRGA_PAR | Drug A Parent | ANALYTE | PLASMA | <0.1 | ng/mL | <0.1 | ng/mL | 0.10 | 20 | 4 | DAY 12 | 12 | 2001-02-12T08:00 | 12 | 24H | 24 | Day 11 Dose | 2001-02-11T08:00 | PT24H |
|
|
