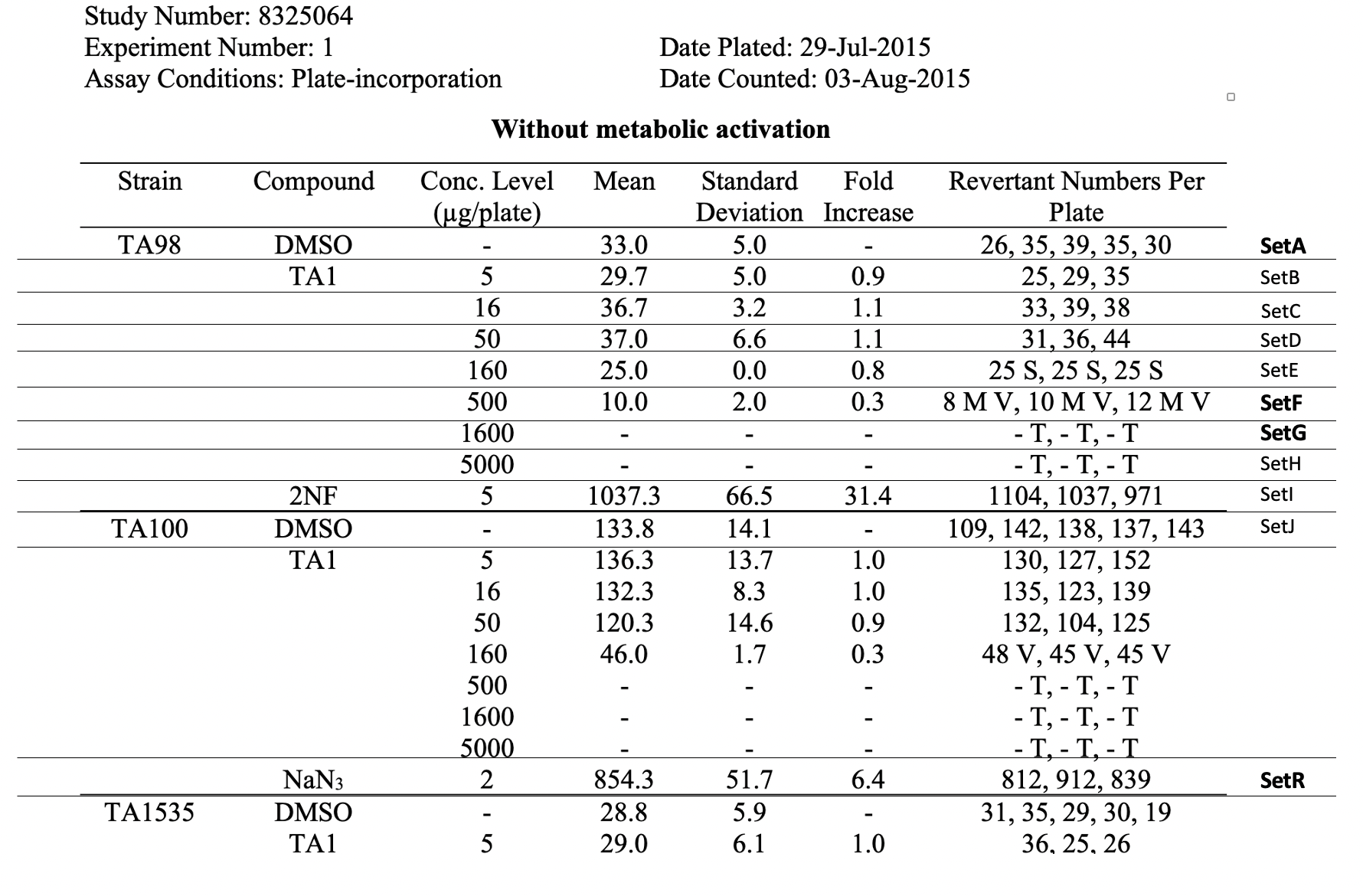
| Row | STUDYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|
1 | | Row | STUDYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|
| 1 | 8325064 | TX | SetA | A-TA98-C0 | 1 | SPECIES | Species | SALMONELLA TYPHIMURIUM |
| 2 | 8325064 | TX | SetA | A-TA98-C0 | 2 | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 |
| 3 | 8325064 | TX | SetA | A-TA98-C0 | 3 | IVTDTRG | In vitro Treatment Duration Target | 72 |
| 4 | 8325064 | TX | SetA | A-TA98-C0 | 4 | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 |
| 5 | 8325064 | TX | SetA | A-TA98-C0 | 5 | IVTDU | In vitro Treatment Duration Unit | HOURS |
| 6 | 8325064 | TX | SetA | A-TA98-C0 | 6 | INCBTMP | Incubation Temperature | 37 |
| 7 | 8325064 | TX | SetA | A-TA98-C0 | 7 | INCBTMPU | Incubation Temperature Unit | C |
| 8 | 8325064 | TX | SetA | A-TA98-C0 | 8 | ATMRHP | Atmospheric Relative Humidity Percent | 50 |
| 9 | 8325064 | TX | SetA | A-TA98-C0 | 9 | ATMCO2P | Atmospheric CO2 Percent | 5 |
| 10 |
1SPECIES | Species | SALMONELLA TYPHIMURIUM | | SPTOBID | Applicant-defined tobacco identifier | CIG01a |
| 11 |
22IVTDMIN | In vitro Treatment Duration Minimum | 71.5 | 33IVTDTRG | In vitro Treatment Duration Target | 72 | | SAMTYP | Sample Type | Total Particulate Matter in PBS |
| 13 |
44IVTDMAX | In vitro Treatment Duration Maximum | 72.5 | | APDEVID | Applicant-defined device identifier | PUFFMASTER3K |
| 14 |
55IVTDU | In vitro Treatment Duration Unit | HOURS | | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN |
| 15 |
66INCBTMP | Incubation Temperature | 37 | | STRAIN | Strain/Substrain | TA98 |
| 16 |
77INCBTMPU | Incubation Temperature Unit | C | | MTACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
| 17 |
88ATMRHP | Atmospheric Relative Humidity Percent | 50 | | METACTFL | Presence of Metabolic Activation Flag | N |
| 18 |
99ATMCO2P | Atmospheric CO2 Percent | 5 | | ITVNAM | Intervention Article Name | DMSO |
| 19 |
1010SPTOBID | Applicant-defined tobacco identifier | CIG01a | | ITVTYPE | Intervention Article Type | VEHICLE |
| 20 |
1111EXPTYP | | Submerged | | ITVCONC | Intervention Article Concentration | 100 |
| 21 |
1212SAMTYP | Sample Type | Total Particulate Matter in PBS | | ITVCONCU | Intervention Article Concentration Unit | % |
| 22 |
1313APDEVID | Applicant-defined device identifier | PUFFMASTER3K | | TRTV | Treatment Vehicle | DMSO |
| 23 |
14SetAAC014SMKRGMSmoking Regimen | NON-INTENSE REGIMEN | | Species | SALMONELLA TYPHIMURIUM |
| 24 |
15SetAAC015STRAIN | Strain/Substrain | TA98 | | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 |
| 25 |
16SetAAC016METACTIND | Metabolic Activating Agent Name | NOT APPLICABLE | | IVTDTRG | In vitro Treatment Duration Target | 72 |
| 26 |
17SetAAC017METACTFL | Presence of Metabolic Activation Flag | N | | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 |
| 27 |
18SetAAC018ITVNAM | Intervention Article Name | DMSO | | IVTDU | In vitro Treatment Duration Unit | HOURS |
| 28 |
19SetAAC019ITVTYPE | Intervention Article Type | VEHICLE | | INCBTMP | Incubation Temperature | 37 |
| 29 |
20SetAAC020ITVCONC | Intervention Article Concentration | 100 | | INCBTMPU | Incubation Temperature Unit | C |
| 30 |
21SetAAC021ITVCONCU | Intervention Article Concentration Unit | % | | ATMRHP | Atmospheric Relative Humidity Percent | 50 |
| 31 |
22SetAAC022TRTV | Treatment Vehicle | DMSO | | ATMCO2P | Atmospheric CO2 Percent | 5 |
| 32 |
2323SPECIES | Species | SALMONELLA TYPHIMURIUM | | SPTOBID | Applicant-defined tobacco identifier | CIG01a |
| 33 |
2424IVTDMIN | In vitro Treatment Duration Minimum | 71.5 | 2525IVTDTRG | In vitro Treatment Duration Target | 72 | | SAMTYP | Sample Type | Total Particulate Matter in PBS |
| 35 |
2626IVTDMAX | In vitro Treatment Duration Maximum | 72.5 | | APDEVID | Applicant-defined device identifier | PUFFMASTER3K |
| 36 |
2727IVTDU | In vitro Treatment Duration Unit | HOURS | | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN |
| 37 |
2828INCBTMP | Incubation Temperature | 37 | | STRAIN | Strain/Substrain | TA98 |
| 38 |
2929INCBTMPU | Incubation Temperature Unit | C | | MTACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
| 39 |
3030ATMRHP | Atmospheric Relative Humidity Percent | 50 | | METACTFL | Presence of Metabolic Activation Flag | N |
| 40 |
3131ATMCO2P | Atmospheric CO2 Percent | 5 | | ITVNAM | Intervention Article Name | TA1 |
| 41 |
3232SPTOBID | Applicant-defined tobacco identifier | CIG01a | | ITVTYPE | Intervention Article Type | PRODUCT |
| 42 |
3333EXPTYP | | Submerged | | ITVCONC | Intervention Article Concentration | 500 |
| 43 |
3434SAMTYP | Sample Type | Total Particulate Matter in PBS | | ITVCONCU | Intervention Article Concentration Unit | ug/plate |
| 44 |
3535APDEVID | Applicant-defined device identifier | PUFFMASTER3K | | TRTV | Treatment Vehicle | DMSO |
| 45 |
36SetFFC50036SMKRGMSmoking Regimen | NON-INTENSE REGIMEN | | Species | SALMONELLA TYPHIMURIUM |
| 46 |
37SetFFC50037STRAIN | Strain/Substrain | TA98 | | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 |
| 47 |
38SetFFC50038METACTIND | Metabolic Activating Agent Name | NOT APPLICABLE | | IVTDTRG | In vitro Treatment Duration Target | 72 |
| 48 |
39SetFFC50039METACTFL | Presence of Metabolic Activation Flag | N | | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 |
| 49 |
40SetFFC50040ITVNAM | Intervention Article Name | TA1 | | IVTDU | In vitro Treatment Duration Unit | HOURS |
| 50 |
41SetFFC50041ITVTYPE | Intervention Article Type | PRODUCT | | INCBTMP | Incubation Temperature | 37 |
| 51 |
42SetFFC50042ITVCONC | Intervention Article Concentration | 500 | | INCBTMPU | Incubation Temperature Unit | C |
| 52 |
43SetFFC50043ITVCONCU | Intervention Article Concentration Unit | ug/plate | | ATMRHP | Atmospheric Relative Humidity Percent | 50 |
| 53 |
44SetFFC50044TRTV | Treatment Vehicle | DMSO | | ATMCO2P | Atmospheric CO2 Percent | 5 |
| 54 |
45 | 8325064 | TX | SetG | G-TA98-C1600 |
45SPECIES | Species | SALMONELLA TYPHIMURIUM | | SPTOBID | Applicant-defined tobacco identifier | CIG01a |
| 55 |
46 | 8325064 | TX | SetG | G-TA98-C1600 |
46IVTDMIN | In vitro Treatment Duration Minimum | 71.5 | 47 | 8325064 | TX | SetG | G-TA98-C1600 |
47IVTDTRG | In vitro Treatment Duration Target | 72 | | SAMTYP | Sample Type | Total Particulate Matter in PBS |
| 57 |
48 | 8325064 | TX | SetG | G-TA98-C1600 |
48IVTDMAX | In vitro Treatment Duration Maximum | 72.5 | | APDEVID | Applicant-defined device identifier | PUFFMASTER3K |
| 58 |
49 | 8325064 | TX | SetG | G-TA98-C1600 |
49IVTDU | In vitro Treatment Duration Unit | HOURS | | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN |
| 59 |
50 | 8325064 | TX | SetG | G-TA98-C1600 |
50INCBTMP | Incubation Temperature | 37 | | STRAIN | Strain/Substrain | TA98 |
| 60 |
51 | 8325064 | TX | SetG | G-TA98-C1600 |
51INCBTMPU | Incubation Temperature Unit | C | | MTACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
| 61 |
52 | 8325064 | TX | SetG | G-TA98-C1600 |
52ATMRHP | Atmospheric Relative Humidity Percent | 50 | | METACTFL | Presence of Metabolic Activation Flag | N |
| 62 |
53 | 8325064 | TX | SetG | G-TA98-C1600 |
53ATMCO2P | Atmospheric CO2 Percent | 5 | | ITVNAM | Intervention Article Name | TA1 |
| 63 |
54 | 8325064 | TX | SetG | G-TA98-C1600 |
54SPTOBID | Applicant-defined tobacco identifier | CIG01a | | ITVTYPE | Intervention Article Type | Product |
| 64 |
55 | 8325064 | TX | SetG | G-TA98-C1600 |
55EXPTYP | | Submerged | | ITVCONC | Intervention Article Concentration | 1600 |
| 65 |
56 | 8325064 | TX | SetG | G-TA98-C1600 |
56SAMTYP | Sample Type | Total Particulate Matter in PBS | | ITVCONCU | Intervention Article Concentration Unit | ug/plate |
| 66 |
57 | 8325064 | TX | SetG | G-TA98-C1600 |
57APDEVID | Applicant-defined device identifier | PUFFMASTER3K | | TRTV | Treatment Vehicle | DMSO |
| 67 |
58SetGGTA98C160058SMKRGMSmoking Regimen | NON-INTENSE REGIMEN | | Species | SALMONELLA TYPHIMURIUM |
| 68 |
59SetGGTA98C160059STRAIN | Strain/Substrain | TA98 | | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 |
| 69 |
60SetGGTA98C160060METACTIND | Metabolic Activating Agent Name | NOT APPLICABLE | | IVTDTRG | In vitro Treatment Duration Target | 72 |
| 70 |
61SetGGTA98C160061METACTFL | Presence of Metabolic Activation Flag | N | | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 |
| 71 |
62SetGGTA98C160062ITVNAM | Intervention Article Name | TA1 | | IVTDU | In vitro Treatment Duration Unit | HOURS |
| 72 |
63SetGGTA98C160063ITVTYPE | Intervention Article Type | Product | | INCBTMP | Incubation Temperature | 37 |
| 73 |
64SetGGTA98C160064ITVCONC | Intervention Article Concentration | 1600 | | INCBTMPU | Incubation Temperature Unit | C |
| 74 |
65SetGGTA98C160065ITVCONCU | Intervention Article Concentration Unit | ug/plate | | ATMRHP | Atmospheric Relative Humidity Percent | 50 |
| 75 |
66SetGGTA98C160066TRTV | Treatment Vehicle | DMSO | | ATMCO2P | Atmospheric CO2 Percent | 5 |
| 76 |
6767SPECIES | Species | SALMONELLA TYPHIMURIUM | | SPTOBID | Applicant-defined tobacco identifier | CIG01a |
| 77 |
6868IVTDMIN | In vitro Treatment Duration Minimum | 71.5 | 6969IVTDTRG | In vitro Treatment Duration Target | 72 | | SAMTYP | Sample Type | Total Particulate Matter in PBS |
| 79 |
7070IVTDMAX | In vitro Treatment Duration Maximum | 72.5 | | APDEVID | Applicant-defined device identifier | PUFFMASTER3K |
| 80 |
7171IVTDU | In vitro Treatment Duration Unit | HOURS | | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN |
| 81 |
7272INCBTMP | Incubation Temperature | 37 | | STRAIN | Strain/Substrain | TA100 |
| 82 |
7373INCBTMPU | Incubation Temperature Unit | C | | MTACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
| 83 |
7474ATMRHP | Atmospheric Relative Humidity Percent | 50 | | METACTFL | Presence of Metabolic Activation Flag | N |
| 84 |
7575ATMCO2P | Atmospheric CO2 Percent | 5 | | ITVNAM | Intervention Article Name | NaN3 |
| 85 |
7676SPTOBID | Applicant-defined tobacco identifier | CIG01a | | ITVTYPE | Intervention Article Type | Positive Control |
| 86 |
| 77 | 8325064 | TX | SetR | R-TA100-C2 | 77 | EXPTYP | | Submerged |
7878 | SAMTYP | Sample Type | Total Particulate Matter in PBS | | 79 | 8325064 | TX | SetR | R-TA100-C2 | 79 | APDEVID | Applicant-defined device identifier | PUFFMASTER3K |
| 80 | 8325064 | TX | SetR | R-TA100-C2 | 80 | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN |
| 81 | 8325064 | TX | SetR | R-TA100-C2 | 81 | STRAIN | Strain/Substrain | TA100 |
| 82 | 8325064 | TX | SetR | R-TA100-C2 | 82 | METACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
| 83 | 8325064 | TX | SetR | R-TA100-C2 | 83 | METACTFL | Presence of Metabolic Activation Flag | N |
| 84 | 8325064 | TX | SetR | R-TA100-C2 | 84 | ITVNAM | Intervention Article Name | NaN3 |
| 85 | 8325064 | TX | SetR | R-TA100-C2 | 85 | ITVTYPE | Intervention Article Type | Positive Control |
| 86 | 8325064 | TX | SetR | R-TA100-C2 | 86 | ITVCONC | Intervention Article Concentration | 2 |
| 87 | 8325064 | TX | SetR | R-TA100-C2 | 87 | ITVCONCU | Intervention Article Concentration Unit | ug/plate |
| 88 | 8325064 | TX | SetR | R-TA100-C2 | 88 | TRTV | Treatment Vehicle | DMSO |