This is an example showing the report table, trial design, and results from a study's in vitro bacterial reverse mutation test
| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-505 |
|---|
|
using 4 different amino acid-requiring strains of
S. typhimurium and 1 strain of
E. coli to detect point mutations.
| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-129 |
|---|
|
| Expand |
|---|
| title | Report Table for Example Study 8325064 |
|---|
|
This table shows the assay results for study 8325064, test article TA1, and 3 strains of salmonella (TA98, TA100, and TA1535) at varying concentrations. For ease of reference, each row has been labeled in the right-brevity, the remaining tables (e.g., additional strains, samples prepared without metabolic activation) are not included. For ease of reference in the table below, each row has been labeled in the right-hand margin with the applicant-defined trial set label (e.g., SetA). For brevity, the remaining tables (e.g., additional strains, samples prepared without metabolic activation) are not included. | Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-506 |
|---|
|
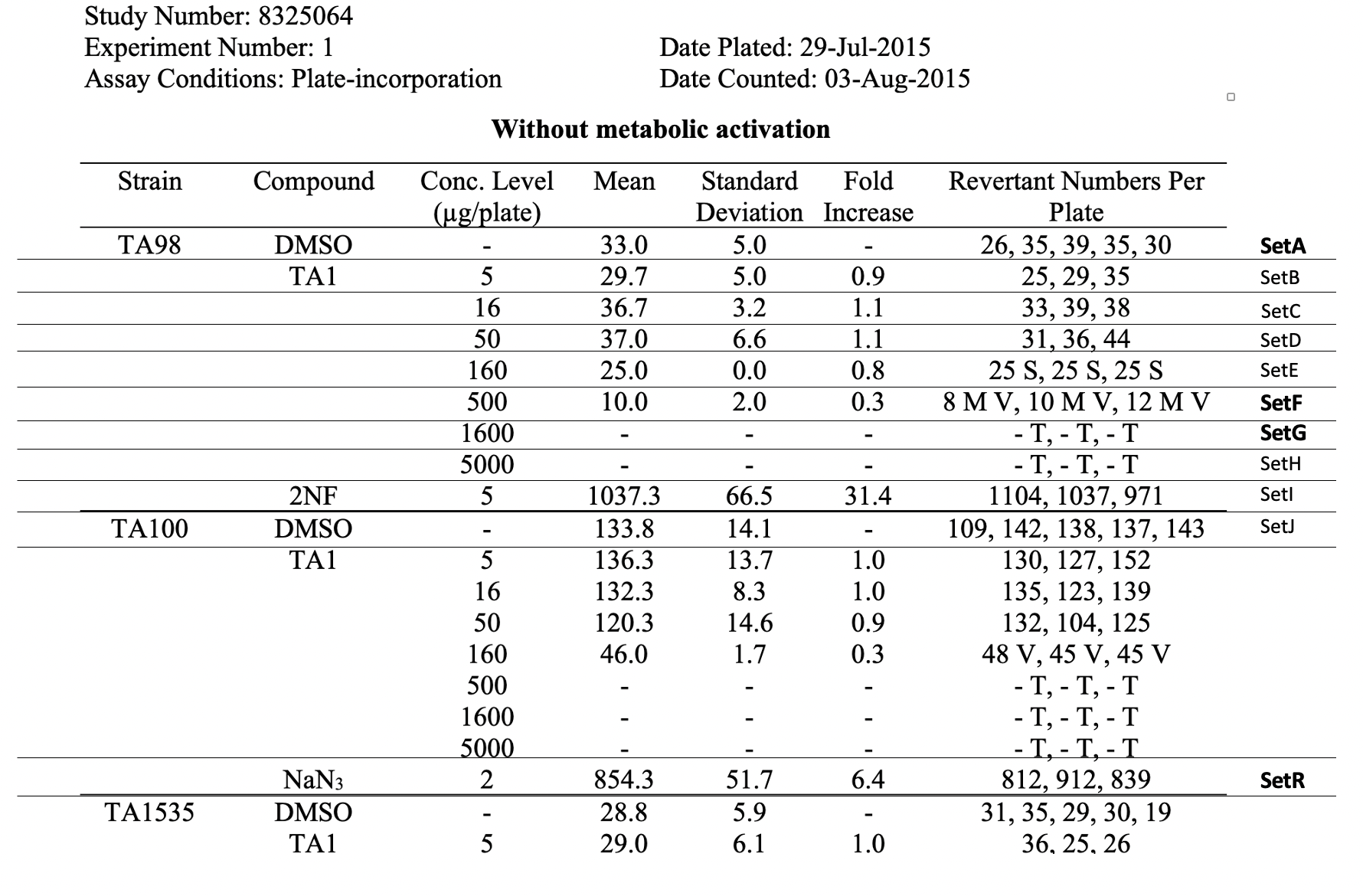 Image Removed Image Removed
 Image Removed Image Removed
|
This example Trial Summary (TS) dataset
| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-481 |
|---|
|
,| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-368 |
|---|
|
shows many informational fields| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-369 |
|---|
|
that provide context at the study level. | Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-663 |
|---|
|
| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-664 |
|---|
|
...
Show 2 records for TSPARMCD = "GLPTYP", using TSSEQ to indicate multiple records,
| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-366 |
|---|
|
since both GLP types apply for this study. ...
Show that TSGRPID (TSGRPID=4) has been used to link the information on the testing guideline followed on this study (TSTGDNAM, TSTGDORG, TSTGDVER).
| Jira |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-56 |
|---|
|
...
Rows 19, 25:
| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-367 |
|---|
|
...
Show that TSGRPID (TSGRPID = 3) has been used to link the information for 1 species (E. coli) with the strain and cell line that is tested in this study.
| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-665 |
|---|
|
only the four bolded sets are represented in the following example datasets.
 Image Added Image Added
 Image Added Image Added
|
This example Trial Summary (TS) dataset, shows many informational fields that provide context at the study level.
| Dataset wrap |
|---|
| Rowcaps |
|---|
| Rows 1-2: | Show 2 records for TSPARMCD = "GLPTYP", using TSSEQ to indicate multiple records, since both GLP types apply for this study. | | Row 3: | Shows that this study was conducted as a GLP study. | | Rows 4-5: | Show the study start date and study title. | | Rows 6-7: | Show the version of SEND Implementation Guide and version of Controlled Terminology used in this study. | | Row 8: | Shows the applicant's organization. | | Row 9: | Shows that the applicant's study reference ID is not applicable. | | Rows 10-13: | Show that TSGRPID has been used to link records (name, location, country) related to the test facility (TSGRPID=1). The study director is associated with the test facility. | | Rows 14-16: | Show that TSGRPID (TSGRPID=4) has been used to link the information on the testing guideline followed on this study (TSTGDNAM, TSTGDORG, TSTGDVER). | | Shows the study type for this study. | | Shows that this study includes a Bacterial Reverse Mutation Assay. | | Rows 19-27: | Show that TSGRPID (TSGRPID = 2) has been used to link the information for 1 species (salmonella) with the 4 different strains and cell lines that are tested in this study. | | Rows 28-30: | Show that TSGRPID (TSGRPID = 3) has been used to link the information for 1 species (E. coli) with the strain and cell line that is tested in this study. |
|
| Dataset2 |
|---|
| Row | STUDYID | DOMAIN | TSSEQ | TSGRPID | TSPARMCD | TSPARM | TSVAL | TSVALNF |
|---|
| 1 | 8325064 | TS | 1 |
| GLPTYP | Good Laboratory Practice Type | FDA |
| | 2 | 8325064 | TS | 2 |
| GLPTYP | Good Laboratory Practice Type | OECD |
| | 3 | 8325064 | TS | 1 |
| GLPFL | GLP Flag | N |
| | 4 | 8325064 | TS | 1 |
| STSTDTC | Study Start Date | 2015-07-29 |
| | 5 | 8325064 | TS | 1 |
| STITLE | Study Title | The Bacterial Reverse Mutation Test, Study 8325064-1 |
| | 6 | 8325064 | TS | 1 |
| SNDIGVER | SEND Implementation Guide Version | TOBACCO IMPLEMENTATION GUIDE VERSION 1.0 |
| | 7 | 8325064 | TS | 1 |
| SNDCTVER | SEND Controlled Terminology Version | SEND Terminology 2021-09-30 |
| | 8 | 8325064 | TS | 1 |
| APPLCNT | Applicant | Example Applicant Inc. |
| | 9 | 8325064 | TS | 1 |
| APREFID | Applicant's Study Reference ID |
| NOT APPLICABLE | | 10 | 8325064 | TS | 1 | 1 | TSTFNAM | Test Facility Name | Example Tox Lab Name |
| | 11 | 8325064 | TS | 1 | 1 | TSTFLOC | Test Facility Location | 10 Somewhere Street, Montgomery, AL 10000 |
| | 12 | 8325064 | TS | 1 | 1 | TFCNTRY | Test Facility Country | USA |
| | 13 | 8325064 | TS | 1 | 1 | STDIR | Study Director | Dr. R. Smith |
| | 14 | 8325064 | TS | 1 | 4 | TSTGDNAM | Testing Guideline Name | Test NO. 471 |
| | 15 | 8325064 | TS | 1 | 4 | TSTGDORG | Testing Guideline Organization | OECD |
| | 16 | 8325064 | TS | 1 | 4 | TSTGDVER | Testing Guideline Version | 2020-06-29 |
| | 17 | 8325064 | TS | 1 |
| SSTYP | Study Type | GENOTOXICITY IN VITRO |
| | 18 | 8325064 | TS | 1 |
| | Genetic Toxicology Assay Identifier | |
| | 19 | 8325064 | TS | 1 | 2 | | Species | |
| | 20 | 8325064 | TS | 1 | 2 | STRAIN | Strain/Substrain | TA98 |
| | 21 | 8325064 | TS | 2 | 2 | STRAIN | Strain/Substrain | TA100 |
| | 22 | 8325064 | TS | 3 | 2 | STRAIN | Strain/Substrain | TA1535 |
| | 23 | 8325064 | TS | 4 | 2 | STRAIN | Strain/Substrain | TA1537 |
| | 24 | 8325064 | TS | 1 | 2 | CELLLN | Cell Line | TA 98 hisD3052; rfa-; uvrB- |
| | 25 | 8325064 | TS | 2 | 2 | CELLLN | Cell Line | TA 100 hisG46; rfa-; uvrB- |
| | 26 | 8325064 | TS | 3 | 2 | CELLLN | Cell Line | TA 1535 hisG46; rfa-; uvrB- |
| | 27 | 8325064 | TS | 4 | 2 | CELLLN | Cell Line | TA 1537 hisC3076; rfa-; uvrB- |
| | 28 | 8325064 | TS | 2 | 3 | SPECIES | Species | |
| | 29 | 8325064 | TS | 5 | 3 | STRAIN | Strain/Substrain | WP2 uvrA pKM101 |
| | 30 | 8325064 | TS | 5 | 3 | CELLLN | Cell Line | trpE uvrA |
|
|
|
This example Trial Set (TX) dataset shows information about the test conditions for SetA and SetR in this study. For brevity, the dataset does not show information for SetF, SetG, or any other sets. A fully formed TX dataset for this example study would include information about the test conditions for all sets.
Note that there are three trial set parameters that link to other important datasets: SPTOBID, APDEVID, and SMKRGM.
- SPTOBID (Applicant-Defined Tobacco Product ID) is used to uniquely identify the tobacco product. The value of SPTOBID (e.g., CIG01a) matches the value for SPTOBID in all the TOPARMCD-TOVAL pairs in the Tobacco Product Identifiers and Descriptors (TO) dataset example in Section 3.1.2, Product Design Parameters and Conformance Testing, Example 1. The TOPARMCD-TOVAL pairs identify this unique product, CIG01a. The TO domain is described in Section 2.8.8.1, Tobacco Product Identifiers and Descriptors (TO).
- The value of APDEVID (e.g., PUFFMASTER3K) matches the value of SPDEVID in all the DIPARMCD-DIVAL pairs that identify this unique device in the DI dataset. SPDEVID is the applicant-defined device identifier that is used to uniquely identify the device in the Unique Device Identification (DI) dataset (see also Section 2.8.9.7, SEND Device Identifiers (DI)). This is shown in the DI dataset in Section 3.1.3.2, HPHCs, Other Constituents, and Smoking/Vaping Regimens, Example 1.
- SMKRGM serves as a link to the Device-In Use Properties (DU) domain (see also Section 2.8.9.8, SEND Device-In-Use (DU)), where a matching value of SMKRGM indicates parameters of the smoking regimen performed by the smoking machine, as in the DU dataset in Section 3.1.3.2, HPHCs, Other Constituents, and Smoking/Vaping Regimens, Example 2.
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Rows 1-22: | Show trial set parameters and trial set values that are the test conditions specific to set SetA which is the vehicle control (i.e., Strain of TA98, vehicle control with concentration value of 0). SetA is associated with the first row, labeled "SetA", in the report table for example study 8325064. | | Rows 23-44: | |
|
|
...
STUDYID
...
DOMAIN
...
TSSEQ
...
TSGRPID
...
TSPARMCD
...
TSPARM
...
TSVAL
...
TSVALNF
...
The Bacterial Reverse Mutation Test, Study 8325064-1
...
SPECIES
| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-484 |
|---|
|
...
SALMONELLA TYPHIMURIUM
| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-123 |
|---|
|
...
Escherichia coli
| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-482 |
|---|
|
...
This example Trial Set (TX) dataset shows information about the test conditions for SetA and SetR in this study. For brevity, the dataset does not show information for SetF, SetG, or any other sets.
| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-483 |
|---|
|
A fully formed TX dataset for this example study would include information about the test conditions for all sets. | Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Rows 1-14: | Show trial set SetSTM, with trial set parameters and trial set values that comprise the test conditions | for all data for the species S. typhimurium. | Rows 15, 24, 33, 42: | Show that SetA, SetF, SetG, and SetR each have a parent set code of SetSTM. As a child set of SetSTM, each of these sets inherit the test conditions of SetSTM. | for the set SetF which is the strain of TA98 with a concentration value of 500 µg/plate. SetF is associated with the sixth row, labeled "SetF", in the report table for example study 8325064. | | Rows 45-66: | Show trial set parameters and trial set values that comprise the test conditions for the set SetG which is the strain of TA98 with a concentration value of 1600 µg/plate. SetG is associated with the seventh row, labeled "SetG", in the report table for example study 8325064. | | Rows 67-88 | Rows 15-23| : | Show trial set parameters and trial set values that comprise the test conditions for the set | SetA. SetA is the data for the vehicle control (i.e., Strain of TA98, vehicle control with concentration value of 0). SetA | SetR which is the strain of TA100, positive control with a concentration value of 2 µg/plate. SetR is associated with the | first | eighteenth row, labeled " | SetA| SetR", in the report table for example study 8325064. | | Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-666 |
|---|
|
| Rows 24-32: | Show trial set parameters and trial set values that comprise the test conditions for the set SetF. SetF is the data for the strain of TA98 with a concentration value of 500 µg/plate. SetF is associated with the sixth row, labeled "SetF", in the report table for example study 8325064. | Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-667 |
|---|
|
| | Rows 33-41: | Show trial set parameters and trial set values that comprise the test conditions for the set SetG. SetG is the data for the strain of TA98 with a concentration value of 1600 µg/plate. SetG is associated with the seventh row, labeled "SetG", in the report table for example study 8325064. | | Rows 42-50: | Show trial set parameters and trial set values that comprise the test conditions for the set SetR. SetR is the data for the strain of TA100, positive control with a concentration value of 2 µg/plate. SetR is associated with the eighteenth row, labeled "SetR", in the report table for example study 8325064. | | Row 10: | SPTOBID (Applicant-Defined Tobacco Product ID) is used to uniquely identify the tobacco product. The value of SPTOBID, in this case CIG01a, matches the value for SPTOBID in all the TOPARMCD-TOVAL pairs in the Tobacco Product Identifiers and Descriptors (TO) dataset example in Section 3.1.2, Product Design Parameters and Conformance Testing, Example 1. The TOPARMCD-TOVAL pairs identify this unique product, CIG01a. The TO domain is described in Section 2.8.8.1, Tobacco Product Identifiers and Descriptors (TO). | | Row 13: | SPDEVID is the applicant-defined device identifier that is used to uniquely identify the device in the Unique Device Identification (DI) dataset (see also Section 2.8.9.7, SEND Device Identifiers (DI)). The value of SPDEVID, in this case PUFFMASTER3K, matches the value of SPDEVID in all the DIPARMCD-DIVAL pairs that identify this unique device in the DI dataset. This is shown in the DI dataset in Section 3.1.3.2, HPHCs, Other Constituents, and Smoking/Vaping Regimens, Example 1. | | Row 14: | Shows a value for SMKRGM. SMKRGM serves as a link to the Device-In Use Properties (DU) domain (see also Section 2.8.9.8, SEND Device-In-Use (DU)), where a matching value of SMKRGM indicates parameters of the smoking regimen performed by the smoking machine, as in the DU dataset in Section 3.1.3.2, HPHCs, Other Constituents, and Smoking/Vaping Regimens, Example 2. | |
|
| Dataset2 |
|---|
| Row | STUDYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|
| 1 | 8325064 | TX | SetA | A-TA98-C0 | 1 | SPECIES | Species | SALMONELLA TYPHIMURIUM | | 2 | 8325064 | TX | SetA | A-TA98-C0 | 2 | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 | | 3 | 8325064 | TX | SetA | A-TA98-C0 | 3 | IVTDTRG | In vitro Treatment Duration Target | 72 | | 4 | 8325064 | TX | SetA | A-TA98-C0 | 4 | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 | | 5 | 8325064 | TX | SetA | A-TA98-C0 | 5 | IVTDU | In vitro Treatment Duration Unit | HOURS | | 6 | 8325064 | TX | SetA | A-TA98-C0 | 6 | INCBTMP | Incubation Temperature | 37 | | 7 | 8325064 | TX | SetA | A-TA98-C0 | 7 | INCBTMPU | Incubation Temperature Unit | C | | 8 | 8325064 | TX | SetA | A-TA98-C0 | 8 | ATMRHP | Atmospheric Relative Humidity Percent | 50 | | 9 | 8325064 | TX | SetA | A-TA98-C0 | 9 | ATMCO2P | Atmospheric CO2 Percent | 5 | | 10 | 8325064 | TX | SetA | A-TA98-C0 | 10 | SPTOBID | Applicant-defined tobacco identifier | CIG01a | | 11 | 8325064 | TX | SetA | A-TA98-C0 | 11 | EXPTYP | | Submerged | | 12 | 8325064 | TX | SetA | A-TA98-C0 | 12 | SAMTYP | Sample Type | Total Particulate Matter in PBS | | 13 | 8325064 | TX | SetA | A-TA98-C0 | 13 | APDEVID | Applicant-defined device identifier | PUFFMASTER3K | | 14 | 8325064 | TX | SetA | A-TA98-C0 | 14 | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN | | 15 | 8325064 | TX | SetA | A-TA98-C0 | 15 | STRAIN | Strain/Substrain | TA98 | | 16 | 8325064 | TX | SetA | A-TA98-C0 | 16 | MTACTIND | Metabolic Activating Agent Name | NOT APPLICABLE | | 17 | 8325064 | TX | SetA | A-TA98-C0 | 17 | METACTFL | Presence of Metabolic Activation Flag | N | | 18 | 8325064 | TX | SetA | A-TA98-C0 | 18 | ITVNAM | Intervention Article Name | DMSO | | 19 | 8325064 | TX | SetA | A-TA98-C0 | 19 | ITVTYPE | Intervention Article Type | VEHICLE | | 20 | 8325064 | TX | SetA | A-TA98-C0 | 20 | ITVCONC | Intervention Article Concentration | 100 | | 21 | 8325064 | TX | SetA | A-TA98-C0 | 21 | ITVCONCU | Intervention Article Concentration Unit | % | | 22 | 8325064 | TX | SetA | A-TA98-C0 | 22 | TRTV | Treatment Vehicle | DMSO | | 23 | 8325064 | TX | SetF | F-TA98-C500 | 23 | SPECIES | Species | SALMONELLA TYPHIMURIUM | | 24 | 8325064 | TX | SetF | F-TA98-C500 | 24 | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 | | 25 | 8325064 | TX | SetF | F-TA98-C500 | 25 | IVTDTRG | In vitro Treatment Duration Target | 72 | | 26 | 8325064 | TX | SetF | F-TA98-C500 | 26 | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 | | 27 | 8325064 | TX | SetF | F-TA98-C500 | 27 | IVTDU | In vitro Treatment Duration Unit | HOURS | | 28 | 8325064 | TX | SetF | F-TA98-C500 | 28 | INCBTMP | Incubation Temperature | 37 | | 29 | 8325064 | TX | SetF | F-TA98-C500 | 29 | INCBTMPU | Incubation Temperature Unit | C | | 30 | 8325064 | TX | SetF | F-TA98-C500 | 30 | ATMRHP | Atmospheric Relative Humidity Percent | 50 | | 31 | 8325064 | TX | SetF | F-TA98-C500 | 31 | ATMCO2P | Atmospheric CO2 Percent | 5 | | 32 | 8325064 | TX | SetF | F-TA98-C500 | 32 | SPTOBID | Applicant-defined tobacco identifier | CIG01a | | 33 | 8325064 | TX | SetF | F-TA98-C500 | 33 | EXPTYP | | Submerged | | 34 | 8325064 | TX | SetF | F-TA98-C500 | 34 | SAMTYP | Sample Type | Total Particulate Matter in PBS | | 35 | 8325064 | TX | SetF | F-TA98-C500 | 35 | APDEVID | Applicant-defined device identifier | PUFFMASTER3K | | 36 | 8325064 | TX | SetF | F-TA98-C500 | 36 | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN | | 37 | 8325064 |
| | Dataset2 |
|---|
| Row | STUDYID | GNTXAID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|
| 1 | 8325064 | Ames | TX | SetSTM | SALMONELLA TYPHIMURIUM | | SPECIES | Species | SALMONELLA TYPHIMURIUM | | 2 | 8325064 | Ames | TX | SetSTM | SALMONELLA TYPHIMURIUM | 2 | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 | | 3 | 8325064 | Ames | TX | SetSTM | SALMONELLA TYPHIMURIUM | 3 | IVTDTRG | In vitro Treatment Duration Target | 72 | | 4 | 8325064 | Ames | TX | SetSTM | SALMONELLA TYPHIMURIUM | 4 | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 | | 5 | 8325064 | Ames | TX | SetSTM | SALMONELLA TYPHIMURIUM | 5 | IVTDU | In vitro Treatment Duration Unit | HOURS | | 6 | 8325064 | Ames | TX | SetSTM | SALMONELLA TYPHIMURIUM | 6 | INCBTMP | Incubation Temperature | 37 | | 7 | 8325064 | Ames | TX | SetSTM | SALMONELLA TYPHIMURIUM | 7 | INCBTMPU | Incubation Temperature Unit | C | | 8 | 8325064 | Ames | TX | SetSTM | SALMONELLA TYPHIMURIUM | 8 | ATMRHP | Atmospheric Relative Humidity Percent | 50 | | 9 | 8325064 | Ames | TX | SetSTM | SALMONELLA TYPHIMURIUM | 9 | ATMCO2P | Atmospheric CO2 Percent | 5 | | 10 | 8325064 | Ames | TX | SetSTM | SALMONELLA TYPHIMURIUM | 10 | SPTOBID | Applicant-defined tobacco identifier | CIG01a | | 11 | 8325064 | Ames | TX | SetSTM | SALMONELLA TYPHIMURIUM | 11 | EXPTYP | | Submerged | | 12 | 8325064 | Ames | TX | SetSTM | SALMONELLA TYPHIMURIUM | 12 | SAMTYP | Sample Type | Total Particulate Matter in PBS | | 13 | 8325064 | Ames | TX | SetSTM | SALMONELLA TYPHIMURIUM | 13 | SPDEVID | Applicant-defined device identifier | PUFFMASTER3K | | 14 | 8325064 | Ames | TX | SetSTM | SALMONELLA TYPHIMURIUM | 14 | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN | | 15 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 15 | PSETCD | Parent Set Code | SetSTM | | 16 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 15 | STRAIN | Strain/Substrain | TA98 | | 17 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 16 | METACTIND | Metabolic Activating Agent Name | NOT APPLICABLE | | 18 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 17 | METACTFL | Presence of Metabolic Activation Flag | N | | 19 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 18 | ITVNAM | Intervention Article Name | TA1 | | 20 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 19 | ITVTYPE | Intervention Article Type | Vehicle Control | | 21 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 20 | ITVCONC | Intervention Article Concentration | 0 | | 22 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 21 | ITVCONCU | Intervention Article Concentration Unit | ug/plate | 23 | 8325064 | Ames | TX | SetA | A-TA98-C0 | TCNTRL | Control Type | VEHICLE CONTROL | | 24 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 22 | TRTV | Treatment Vehicle | DMSO | 25 | 8325064 | Ames | TX | SetF | F-TA98-C500 | Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-485 |
|---|
|
| PSETCD | Parent Set Code | SetSTM | 26 | 8325064 | Ames23| 37 | STRAIN | Strain/Substrain | TA98 |
27Ames24METACTIND| MTACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
28Ames | 25| 39 | METACTFL | Presence of Metabolic Activation Flag | N |
29Ames26| 40 | ITVNAM | Intervention Article Name | TA1 |
30Ames27| 41 | ITVTYPE | Intervention Article Type |
Product31Ames | 28| 42 | ITVCONC | Intervention Article Concentration | 500 |
32Ames29| 43 | ITVCONCU | Intervention Article Concentration Unit | ug/plate |
33Ames30| 44 | TRTV | Treatment Vehicle | DMSO |
34Ames31PSETCDParent Set Code | SetSTM | 35| Species | SALMONELLA TYPHIMURIUM | | 46 | 8325064 |
Ames31STRAIN | Strain/Substrain | TA98 | 36 | 8325064 | | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 | | 47 | 8325064 |
Ames32METACTIND | Metabolic Activating Agent Name | NOT APPLICABLE | | IVTDTRG | In vitro Treatment Duration Target | 72 | | 48 | 8325064 |
37 | 8325064 | Ames33METACTFL | Presence of Metabolic Activation Flag | N | | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 | | 49 |
38Ames | 34ITVNAM | Intervention Article Name | TA1 | | IVTDU | In vitro Treatment Duration Unit | HOURS | | 50 | 8325064 |
39 | 8325064 | Ames35ITVTYPE | Intervention Article Type | Product | | INCBTMP | Incubation Temperature | 37 | | 51 | 8325064 |
40 | 8325064 | Ames36ITVCONC | Intervention Article Concentration | 1600 | | INCBTMPU | Incubation Temperature Unit | C | | 52 |
41Ames | 37ITVCONCU | Intervention Article Concentration Unit | ug/plate | | ATMRHP | Atmospheric Relative Humidity Percent | 50 | | 53 |
42Ames | 38TRTV | Treatment Vehicle | DMSO | | ATMCO2P | Atmospheric CO2 Percent | 5 | | 54 |
43Ames | SetRRTA100C239PSETCD | Parent Set Code | SetSTM | | SPTOBID | Applicant-defined tobacco identifier | CIG01a | | 55 |
44Ames | SetRRTA100C239STRAIN | Strain/Substrain | TA100 | 45 | 8325064 | AmesSetRRTA100C240METACTIND | Metabolic Activating Agent Name | NOT APPLICABLE | | SAMTYP | Sample Type | Total Particulate Matter in PBS | | 57 | 8325064 |
46 | 8325064 | AmesSetRRTA100C241METACTFL | Presence of Metabolic Activation Flag | N | | APDEVID | Applicant-defined device identifier | PUFFMASTER3K | | 58 |
47Ames | SetRRTA100C242ITVNAM | Intervention Article Name | NaN3 | 48 | 8325064 | | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN | | 59 | 8325064 |
AmesSetRRTA100C243ITVTYPE | Intervention Article Type | Positive Control | | STRAIN | Strain/Substrain | TA98 | | 60 | 8325064 |
49 | 8325064 | AmesSetRRTA100C244ITVCONC | Intervention Article Concentration | 2 | | MTACTIND | Metabolic Activating Agent Name | NOT APPLICABLE | | 61 | 8325064 |
50 | 8325064 | AmesSetRRTA100C245ITVCONCU | Intervention Article Concentration Unit | ug/plate | 51 | 8325064 | Ames | TX | SetR | R-TA100-C2 | TCNTRL | Control Type | POSITIVE CONTROL | | 52 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 46 | TRTV | Treatment Vehicle | DMSO | |
|
REFID values are defined by the applicant to uniquely identify the observational unit within an experimental unit. In the simplified diagram of an assay procedure (below), the test tube that contains the possible mutagen is an example of an entity that would have a REFID indicating information at the level of trial set. The petri plate created from that test tube and used to count revertant numbers is an example of an entity that would be at an observational level.
This picture does not depict the more complicated procedures,
| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-370 |
|---|
|
trial sets, or observational units for the example study. For study 8325064, the applicant chose to define the REFIDs at the trial set level with a single character (e.g., A, F, G, R) and chose to define REFIDs at the observational level based on the location of each tube in 1 of multiple exposure/incubation plates (e.g, 6_1 for plate 6, position 1). Image Removed
Image Removed
| METACTFL | Presence of Metabolic Activation Flag | N | | 62 | 8325064 | TX | SetG | G-TA98-C1600 | 62 | ITVNAM | Intervention Article Name | TA1 | | 63 | 8325064 | TX | SetG | G-TA98-C1600 | 63 | ITVTYPE | Intervention Article Type | Product | | 64 | 8325064 | TX | SetG | G-TA98-C1600 | 64 | ITVCONC | Intervention Article Concentration | 1600 | | 65 | 8325064 | TX | SetG | G-TA98-C1600 | 65 | ITVCONCU | Intervention Article Concentration Unit | ug/plate | | 66 | 8325064 | TX | SetG | G-TA98-C1600 | 66 | TRTV | Treatment Vehicle | DMSO | | 67 | 8325064 | TX | SetR | R-TA100-C2 | 67 | SPECIES | Species | SALMONELLA TYPHIMURIUM | | 68 | 8325064 | TX | SetR | R-TA100-C2 | 68 | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 | | 69 | 8325064 | TX | SetR | R-TA100-C2 | 69 | IVTDTRG | In vitro Treatment Duration Target | 72 | | 70 | 8325064 | TX | SetR | R-TA100-C2 | 70 | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 | | 71 | 8325064 | TX | SetR | R-TA100-C2 | 71 | IVTDU | In vitro Treatment Duration Unit | HOURS | | 72 | 8325064 | TX | SetR | R-TA100-C2 | 72 | INCBTMP | Incubation Temperature | 37 | | 73 | 8325064 | TX | SetR | R-TA100-C2 | 73 | INCBTMPU | Incubation Temperature Unit | C | | 74 | 8325064 | TX | SetR | R-TA100-C2 | 74 | ATMRHP | Atmospheric Relative Humidity Percent | 50 | | 75 | 8325064 | TX | SetR | R-TA100-C2 | 75 | ATMCO2P | Atmospheric CO2 Percent | 5 | | 76 | 8325064 | TX | SetR | R-TA100-C2 | 76 | SPTOBID | Applicant-defined tobacco identifier | CIG01a | | 77 | 8325064 | TX | SetR | R-TA100-C2 | 77 | EXPTYP | | Submerged | | 78 | 8325064 | TX | SetR | R-TA100-C2 | 78 | SAMTYP | Sample Type | Total Particulate Matter in PBS | | 79 | 8325064 | TX | SetR | R-TA100-C2 | 79 | APDEVID | Applicant-defined device identifier | PUFFMASTER3K | | 80 | 8325064 | TX | SetR | R-TA100-C2 | 80 | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN | | 81 | 8325064 | TX | SetR | R-TA100-C2 | 81 | STRAIN | Strain/Substrain | TA100 | | 82 | 8325064 | TX | SetR | R-TA100-C2 | 82 | MTACTIND | Metabolic Activating Agent Name | NOT APPLICABLE | | 83 | 8325064 | TX | SetR | R-TA100-C2 | 83 | METACTFL | Presence of Metabolic Activation Flag | N | | 84 | 8325064 | TX | SetR | R-TA100-C2 | 84 | ITVNAM | Intervention Article Name | NaN3 | | 85 | 8325064 | TX | SetR | R-TA100-C2 | 85 | ITVTYPE | Intervention Article Type | Positive Control | | 86 | 8325064 | TX | SetR | R-TA100-C2 | 86 | ITVCONC | Intervention Article Concentration | 2 | | 87 | 8325064 | TX | SetR | R-TA100-C2 | 87 | ITVCONCU | Intervention Article Concentration Unit | ug/plate | | 88 | 8325064 | TX | SetR | R-TA100-C2 | 88 | TRTV | Treatment Vehicle | DMSO |
|
|
REFID values are defined by the applicant to uniquely identify the observational unit within an experimental unit. In the simplified diagram of an assay procedure (below), the test tube that contains the possible mutagen is an example of an entity that would have a REFID indicating information at the level of trial set. The petri plate created from that test tube and used to count revertant numbers is an example of an entity that would be at an observational level.
This picture does not depict the more complicated procedures, trial sets, or observational units for the example study. For study 8325064, the applicant chose to define the REFIDs at the trial set level with a single character (e.g., A, F, G, R) and chose to define REFIDs at the observational level based on the location of each tube in 1 of multiple exposure/incubation plates (e.g, 6_1 for plate 6, position 1).
 Image Added
Image Added
| Dataset wrap |
|---|
|
|
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Row 1: | Shows the value of REFID=STM. This REFID refers to the trial set with a SETCD of "SetSTM" | Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-489 |
|---|
|
, as defined in the TX dataset. LEVEL=1 and LVLDESC="TRIAL SET" indicates this identifier relates to the entire trial set. | | Rows 1, 2, 8, 12, 16: | Show that the trial set SetSTM is the parent of set codes SetA, SetF, SetG, and SetR. | | Row 2: | Shows the value of REFID=A. This REFID refers to the trial set with a SETCD of "SetA", as defined in the TX dataset. LEVEL= 1 and LVLDESC="EXPERIMENTAL UNIT/TRIAL SET". TRIAL SET indicates this identifier relates to the entire trial set. EXPERIMENTAL UNIT indicates this identifier received the intervention article, DMSO. | | Rows 32-76: | Show the values of 5 observational units (1_1 through 1_5) that are within the parent experimental unit, REFID=A. | | Row 87: | Shows the value of REFID=F. This REFID refers to the trial set with a SETCD of "SetF", as defined in the TX dataset. LEVEL=2 1 and LVLDESC="EXPERIMENTAL UNIT/TRIAL SET". TRIAL SET indicates this identifier relates to the entire trial set. EXPERIMENTAL UNIT indicates this identifier received the intervention article, TA1. | | Rows 98-1110: | Show the values of 3 observational units (6_1 through 6_3) that are within the parent experimental unit, REFID=F. | | Row 1211: | Shows the value of REFID=G. This REFID refers to the trial set with a SETCD of "SetG", as defined in the TX dataset. LEVEL=2 1 and LVLDESC="EXPERIMENTAL UNIT/TRIAL SET". TRIAL SET indicates this identifier relates to the entire trial setthe entire trial set. EXPERIMENTAL UNIT indicates this identifier received the intervention article, TA1. | | Rows 1312-1514: | Show the values of 3 observational units (7_1 through 7_3) that are within the parent experimental unit, REFID=G. | | Row 1615: | Shows the value of REFID=R. This REFID refers to the trial set with a SETCD of "SetG", as defined in the TX dataset. LEVEL=2 1 and LVLDESC="EXPERIMENTAL UNIT/TRIAL SET". TRIAL SET indicates this identifier relates to the entire trial set. EXPERIMENTAL UNIT indicates this identifier received the intervention article, NaN3. | | Rows 1716-1918: | Show the values of 3 observational units (18_1 through 18_3) that are within the parent experimental unit, REFID=R.| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
key | TOBA-668 |
|
| Dataset2 |
|---|
| Row | STUDYID |
|---|
GNTXAID | SETCD | REFID | PARENT | LEVEL | LVLDESC | 1 | 8325064 | Ames | SetSTM | STM | 1 | TRIAL SET | | SETCD | REFID | PARENT | LEVEL | LVLDESC |
|---|
1 | 8325064 |
|---|
2 | 8325064 | Ames2 | SetA | A | STM |
| 1 | EXPERIMENTAL UNIT/TRIAL SET | 32 | 8325064 |
|---|
Ames | SetA | 1_1 | A | 32 | OBSERVATIONAL UNIT | 43 | 8325064 |
|---|
Ames | SetA | 1_2 | A | 32 | OBSERVATIONAL UNIT | 54 | 8325064 |
|---|
Ames | SetA | 1_3 | A | 32 | OBSERVATIONAL UNIT | 65 | 8325064 |
|---|
Ames | SetA | 1_4 | A | 32 | OBSERVATIONAL UNIT | 76 | 8325064 |
|---|
Ames | SetA | 1_5 | A | 32 | OBSERVATIONAL UNIT | 87 | 8325064 |
|---|
Ames | SetF | F | STM
| 1 | 2 | EXPERIMENTAL UNIT/TRIAL SET | 98 | 8325064 |
|---|
Ames | SetF | 6_1 | F | 32 | OBSERVATIONAL UNIT | 109 | 8325064 |
|---|
Ames | | SetF | 6_2 | F | 32 | OBSERVATIONAL UNIT | 1110 | 8325064 |
|---|
Ames | SetF | 6_3 | F | 32 | OBSERVATIONAL UNIT | 1211 | 8325064 |
|---|
Ames | SetG | G | STM
| 1 | 2 | EXPERIMENTAL UNIT/TRIAL SET | 1312 | 8325064 |
|---|
Ames | | SetG | 7_1 | G | 32 | OBSERVATIONAL UNIT | 1413 | 8325064 |
|---|
Ames | SetG | 7_2 | G | 32 | OBSERVATIONAL UNIT | 15| 14 | 8325064 | Ames | SetG | 7_3 | G | 32 | OBSERVATIONAL UNIT | 16| 15 | 8325064 | AmesSetR | SetG | 2R | STM |
| 1 | EXPERIMENTAL UNIT/TRIAL SET | 17| 16 | 8325064 | Ames | SetR | 18_1 | R | 32 | OBSERVATIONAL UNIT | 18| 17 | 8325064 | Ames | SetR | 18_2 | R | 32 | OBSERVATIONAL UNIT | 19| 18 | 8325064 | Ames | | SetR | 18_3 | R | 3 | OBSERVATIONAL UNIT |
|
|
...
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Rows 1-5: | Show the number of revertant colonies per plate collected for each of 5 observational units, GTREFID=1_1 through 1_5 (see description in the RELREF dataset). | | Rows 6, 7: | Show summary values collected (mean, standard deviation) for GTREFID=A that apply to the entire trial set, SetA, as indicated by LEVEL=1 and LVLDESC=TRIAL SET for this REFID as shown in the RELREF dataset. | | Rows 8-13: | Revertent colonies were counted for each of 3 plates/observational units (GTREFID=6_1 through 6_3) and each value is associated with a record to show a postfix code of "V" (Very thin background bacterial lawn). | | Rows 14-16: | Show summary values collected (mean, standard deviation, fold increase) for GTREFID=F that apply to the entire trial set, SetF, as indicated by LEVEL=1 and LVLDESC=TRIAL SET for this REFID in the RELREF dataset. | | Rows 17-19: | Show 3 plates/observational units (GTREFID=7_1 through 7_3) where no revertant colonies were counted due to too much cytotoxicity and a postfix code of "T". | Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| columnIds | issuekey,summary,issuetype,created,updated,duedate,assignee,reporter,priority,status,resolution |
|---|
| columns | key,summary,type,created,updated,due,assignee,reporter,priority,status,resolution |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-668 |
|---|
|
| | Rows 20-22: | Show the number of revertant colonies per plate collected for each of 3 observational units (GTREFID=18_1 through 18_3). | | Rows 23-25: | Show summary values collected (mean, standard deviation, fold increase) for GTREFID=R that apply to the entire trial set, SetR, as indicated by LEVEL=1 and LVLDESC=TRIAL SET for this REFID in the RELREF dataset. |
|
| Dataset2 |
|---|
| GNTXAID | | DOMAIN | GTSEQ | GTREFID | GTTESTCD | GTTEST | GTORRES | GTORRESU | GTCOLSRT | GTSTRESC | GTSTRESN | GTSTRESU | GTSTAT | GTREASND | GTMETHOD | GTDTC |
|---|
| 1 | 8325064 |
Ames | GT | 1 | 1_1 | RPP | Revertant Colony Numbers Per Plate | 26 |
NOT APPLICABLE | NOT APPLICABLE| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
key | TOBA-487 | |
|
| INSTRUMENT COUNTED | 2015-08-03 | | 2 | 8325064 |
Ames | GT | 2 | 1_2 | RPP | Revertant Colony Numbers Per Plate | 35 |
NOT APPLICABLE | NOT APPLICABLE |
|
|
| INSTRUMENT COUNTED | 2015-08-03 | | 3 | 8325064 |
Ames | | GT | 3 | 1_3 | RPP | Revertant Colony Numbers Per Plate | 39 |
NOT APPLICABLENOT APPLICABLE |
|
|
| INSTRUMENT COUNTED | 2015-08-03 | | 4 | 8325064 |
Ames | GT | 4 | 1_4 | RPP | Revertant Colony Numbers Per Plate | 35 |
NOT APPLICABLE | NOT APPLICABLE |
|
|
| INSTRUMENT COUNTED | 2015-08-03 | | 5 | 8325064 |
Ames | | GT | 5 | 1_5 | RPP | Revertant Colony Numbers Per Plate | 30 |
NOT APPLICABLENOT APPLICABLE |
|
|
| INSTRUMENT COUNTED | 2015-08-03 | | 6 | 8325064 |
Ames | GT | 6 | A | RPP | Revertant Colony Numbers Per Plate | 33.0 |
NOT APPLICABLENOT APPLICABLE | Ames | GT | 7 | A | RPP | Revertant Colony Numbers Per Plate | 5.0 |
NOT APPLICABLE | NOT APPLICABLEAmes | 1| 8 | 6_1 | RPP | Revertant Colony Numbers Per Plate | 8 |
NOT APPLICABLE | NOT APPLICABLE |
|
|
| MANUALLY COUNTED | 2015-08-03 | | 9 | 8325064 |
Ames | 2| 9 | 6_1 | CYTOTOX | Cytotoxicity | Very thin background bacterial lawn |
|
| V |
|
|
|
|
| 2015-08-03 | | 10 | 8325064 |
Ames3| 10 | 6_2 | RPP | Revertant Colony Numbers Per Plate | 10 |
NOT APPLICABLENOT APPLICABLE |
|
|
| MANUALLY COUNTED | 2015-08-03 | | 11 | 8325064 |
Ames4| 11 | 6_2 | CYTOTOX | Cytotoxicity | Very thin background bacterial lawn |
|
| V |
|
|
|
|
| 2015-08-03 | | 12 | 8325064 |
Ames5| 12 | 6_3 | RPP | Revertant Colony Numbers Per Plate | 12 |
NOT APPLICABLENOT APPLICABLE |
|
|
| MANUALLY COUNTED | 2015-08-03 | | 13 | 8325064 |
Ames6| 13 | 6_3 | CYTOTOX | Cytotoxicity | Very thin background bacterial lawn |
|
| V |
|
|
|
|
| 2015-08-03 | | 14 | 8325064 |
Ames7| 14 | F | RPPMEAN | Mean Rev Colony Num Per Plate | 10.0 |
NOT APPLICABLENOT APPLICABLE | Ames8| 15 | F | RPPSTDDV | Std Dev Rev Colony Num Per Plate | 2.0 |
NOT APPLICABLENOT APPLICABLE | Ames9| 16 | F | RPPFLDIC | Fold Increase Rev Colony Num Per Plate | 0.3 |
NOT APPLICABLENOT APPLICABLE | Ames1| 17 | 7_1 | CYTOTOX | Cytotoxicity | Toxic No Revertant Colonies |
|
| T |
|
| NOT DONE | TOO MUCH CYTOTOXICITY |
| 2015-08-03 | | 18 | 8325064 |
Ames | 2| 18 | 7_2 | CYTOTOX | Cytotoxicity | Toxic No Revertant Colonies |
|
| T |
|
| NOT DONE | TOO MUCH CYTOTOXICITY |
| 2015-08-03 | | 19 | 8325064 |
Ames | 3| 19 | 7_3 | CYTOTOX | Cytotoxicity | Toxic No Revertant Colonies |
|
| T |
|
| NOT DONE | TOO MUCH CYTOTOXICITY |
| 2015-08-03 | | 20 | 8325064 |
Ames1| 20 | 18_1 | RPP | Revertant Colony Numbers Per Plate | 812 |
NOT APPLICABLENOT APPLICABLE |
|
|
| INSTRUMENT COUNTED | 2015-08-03 | | 21 | 8325064 |
Ames2| 21 | 18_2 | RPP | Revertant Colony Numbers Per Plate | 912 |
NOT APPLICABLENOT APPLICABLE |
|
|
| INSTRUMENT COUNTED | 2015-08-03 | | 22 | 8325064 |
Ames3| 22 | 18_3 | RPP | Revertant Colony Numbers Per Plate | 839 |
NOT APPLICABLENOT APPLICABLE |
|
|
| INSTRUMENT COUNTED | 2015-08-03 | | 23 | 8325064 |
Ames4| 23 | R | RPPMEAN | Mean Rev Colony Num Per Plate | 854.3 |
NOT APPLICABLENOT APPLICABLEAmes5| 24 | R | RPPSTDDV | Std Dev Rev Colony Num Per Plate | 51.7 |
NOT APPLICABLE |
| STANDARD DEVIATION | 51.7 | 51.7 |
NOT APPLICABLEAmes6| 25 | R | RPPFLDIC | Fold Increase Rev Colony Num Per Plate | 6.4 |
NOT APPLICABLENOT APPLICABLE |
|


