This example shows a sample report table, trial design, and results dataset for study 123 for the determination of the in vitro genotoxicity potential of tobacco products using the in vitro micronucleus assay. | Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
serverId
85506ce4-3cb3-3d91-85ee-f633aaaf4a45 | | key | TOBA-642 |
|---|
| Expand |
|---|
| title | Sample Report Table for Study 123 |
|---|
|
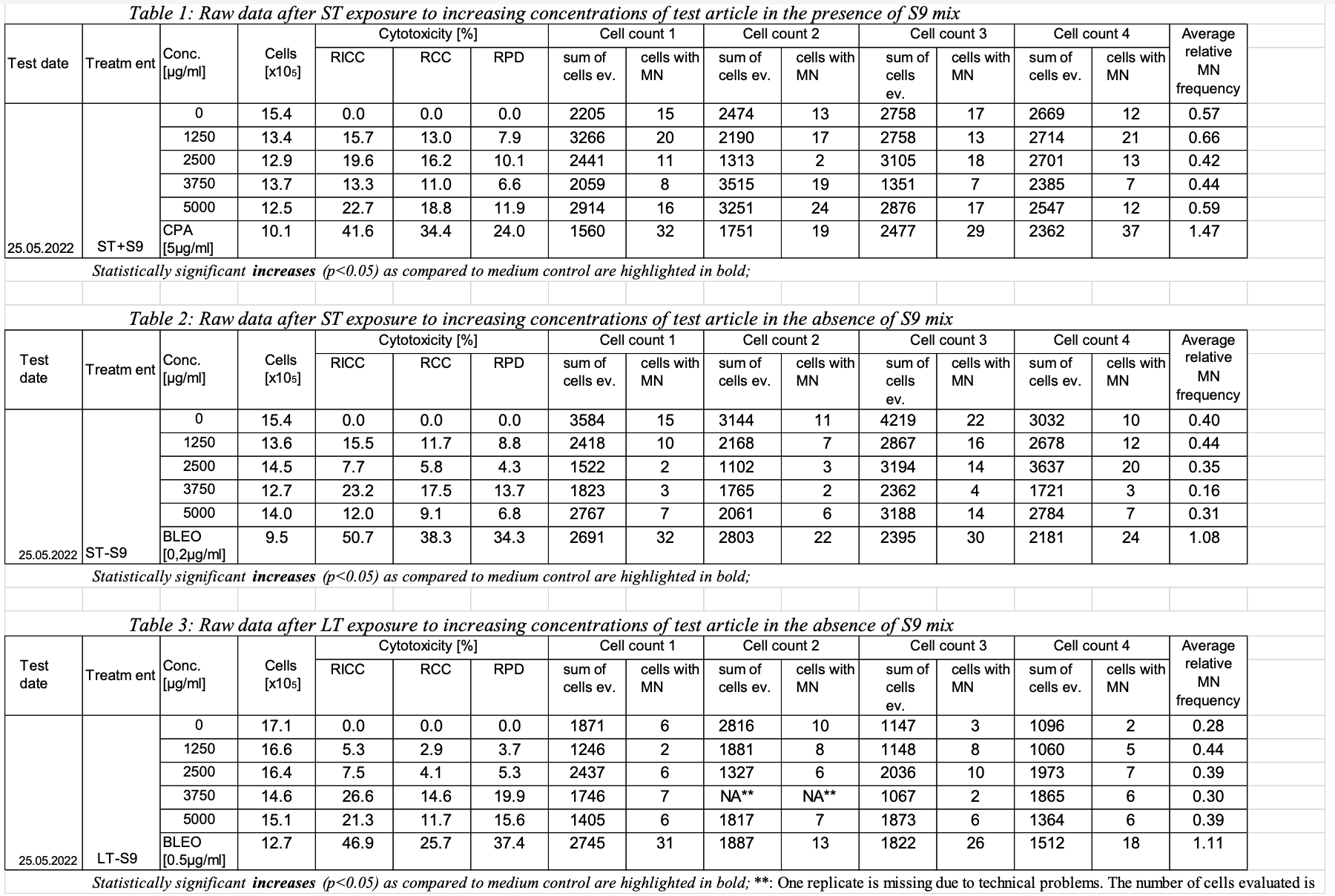
|
...
...
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-456 |
|---|
| Rowcaps |
|---|
| Rows 1-2: | Show 2 records for TSPARMCD = |
|
|
| Dataset wrap |
|---|
| Rows 1-2: | Show 2 records for TSPARMCD = | "GLPTYP", using TSSEQ to indicate multiple records, |
|
 Image RemovedTOBA-366 UNDER GGG REVIEW
Image RemovedTOBA-366 UNDER GGG REVIEW | since both GLP types apply for |
|
this | this example study. | | Row 3: | Shows that this study was conducted as a GLP study. | | Rows 4-5: |
|
Shows | Show the study start date and study title. | | Rows 6-7: | Show the version of SEND Implementation Guide and version of Controlled Terminology used in this study. | | Row 8: | Shows the applicant's organization. | | Row 9: | Shows that the applicant' |
|
s study | s study reference ID is not applicable. | | Rows 10-13: | Show that TSGRPID has been used to link records (name, location, country) related to the test facility (TSGRPID = 1). The study director is associated with the test facility. | | Rows 14-16: | Show that TSGRPID (TSGRPID= |
|
42) has been used to link the information on the testing guideline followed on this study (TSTGDNAM, TSTGDORG, TSTGDVER). |
|
 Image RemovedTOBA-56 - Assay Standard DONERow 17
Image RemovedTOBA-56 - Assay Standard DONERow 17 | Shows the study type for this study. | | Shows that this study includes a Mammalian Cell Micronucleus Assay. | | Rows 19- |
|
27 TSGRPID (TSGRPID = 2) has been used to link information for 1 species (salmonella) with the 4 different strains and cell lines that are tested in this study.| species is human and the cell line is TK6 lymphoblastoid in this study. |
|
| Dataset2 |
|---|
| Row | STUDYID | DOMAIN | TSSEQ | TSGRPID | TSPARMCD | TSPARM | TSVAL | TSVALNF |
|---|
| 1 | 123 | TS | 1 |
| GLPTYP | Good Laboratory Practice Type | FDA |
| | 2 | 123 | TS | 2 |
| GLPTYP | Good Laboratory Practice Type | OECD |
| | 3 | 123 | TS | 1 |
| GLPFL | GLP Flag | Y |
| | 4 | 123 | TS | 1 |
| STSTDTC | Study Start Date | 2022-05-25 |
| | 5 | 123 | TS | 1 |
| STITLE | Study Title | Determination of the in vitro genotoxicity potential using the in vitro Neutral Red Uptake assay |
| | 6 | 123 | TS | 1 |
| SNDIGVER | SEND Implementation Guide Version | TOBACCO IMPLEMENTATION GUIDE VERSION 1.0 |
| | 7 | 123 | TS | 1 |
| SNDCTVER | SEND Controlled Terminology Version | SEND Terminology 2021-09-30 |
| | 8 | 123 | TS | 1 |
| APPLCNT | | Example Applicant, Inc. |
| | 9 |
|
| Rows 28-30: | Show that TSGRPID (TSGRPID = 3) has been used to link the information for 1 species (E. coli) with the strain and cell line that is tested in this study.  Image RemovedTOBA-665 UNDER G Image RemovedTOBA-665 UNDER G |
| Rowcaps |
|---|
| Rows 1-2: | Show 2 records for TSPARMCD = "GLPTYP", using TSSEQ to indicate multiple records, since both GLP types apply for this example study. |
| Row 3: | Shows that this study was conducted as a GLP study. |
| Rows 4-5: | Shows the study start date and study title. |
| Rows 6-7: | Show the version of SEND Implementation Guide and version of Controlled Terminology used in this study. |
| Row 8: | Shows the applicant's organization. |
| Row 9: | Shows that the applicant's study reference ID is not applicable. |
| Rows 10-13: | Show that TSGRPID has been used to link records (name, location, country) related to the test facility (TSGRPID = 1). The study director is associated with the test facility. |
| Rows 14-16: | Show that TSGRPID (TSGRPID=2) has been used to link the information on the testing guideline followed on this study (TSTGDNAM, TSTGDORG, TSTGDVER). | Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-56 |
|---|
|
|
| Shows the study type for this study. |
| Shows that this study includes a Mammalian Cell Micronucleus Assay. |
| Rows 19-20: | Show that the species is human and the cell line is TK6 lymphoblastoid in this study. |
| Dataset2 |
|---|
| Row | STUDYID | DOMAIN | TSSEQ | TSGRPID | TSPARMCD | TSPARM | TSVAL | TSVALNF |
|---|
| 1 | 123 | TS | 1 | GLPTYP | Good Laboratory Practice Type | FDA | 2 | 123 | TS | 2 | GLPTYP | Good Laboratory Practice Type | OECD | 3 | 123 | TS | 1 |
| APREFID | Study Reference ID |
| NOT APPLICABLE | | 10 | GLPFL | GLP Flag | Y | 4 | 123 | TS | 1 | STSTDTC1 | Study Start Date | 2022-05-25 | TSTFNAM | Test Facility Name | Example Test Lab Name |
| | 11 | 5 | 123 | TS | 1 | 1 | TSTFLOC | Test Facility Location | 10 Somewhere Street, Montgomery, AL 10000 |
| | 12 | STITLE | Study Title | Determination of the in vitro genotoxicity potential using the in vitro Neutral Red Uptake assay | 6 | 123 | TS | 1 | 1 | TFCNTRY | Test Facility Country | USA |
| | 13 | SNDIGVER | SEND Implementation Guide Version | TOBACCO IMPLEMENTATION GUIDE VERSION 1.0 | 7 | 123 | TS | 1 | SNDCTVER | SEND Controlled Terminology Version | 1 | STDIR | Study Director | Dr. R. Smith |
| | 14 | SEND Terminology 2021-09-30 | 8 | 123 | TS | 1 | 2 | TSTGDNAM | Testing Guideline Name | GUIDELINE FOR THE TESTING OF CHEMICALS No. 487 |
| | 15 | 123 | TS | 1 | 2 | TSTGDORG | Testing Guideline Organization | OECD |
| | 16 | APPLCNT | Applicant Organization | Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-356 |
|---|
|
| Example Applicant, Inc. | 9 | 123 | TS | 1 | 2 | TSTGDVER | Testing Guideline Version | 29-July-2016 |
| | 17 | APREFID | Study Reference ID | NOT APPLICABLE | 10 | 123 | TS | 11 |
| SSTYP | TSTFNAM | Test Facility Name | Example Test Lab Name | Study Type | GENOTOXICITY IN VITRO |
| | 18 | 11 | 123 | TS | 1 | 1 | TSTFLOC | Test Facility Location |
| GNTXAID | Genetic Toxicology Assay Identifier | MNvit |
| | 19 | 10 Somewhere Street, Montgomery, AL 10000 | 12 | 123 | TS | 11 |
| SPECIES | TFCNTRYSpecies | Test Facility Country | USA | HUMAN |
| | 2013 | 123 | TS | 11 |
| CELLLN | STDIR | Study Director | Dr. R. Smith | 14 | 123 | TS | 1 | 2 | TSTGDNAM | Testing Guideline Name | GUIDELINE FOR THE TESTING OF CHEMICALS No. 487 | 15 | 123 | TS | 1 | 2 | TSTGDORG | Testing Guideline Organization | OECD | 16 | 123 | TS | 1 | 2 | TSTGDVER | Testing Guideline Version | 29-July-2016 | 17 | 123 | TS | 1 | SSTYP | Study Type | GENOTOXICITY IN VITRO | 18 | 123 | TS | 1 | GNTXAID | Genetic Toxicology Assay Identifier | MNvit | 19 | 123 | TS | 1 | SPECIES | Species | HUMAN | 20 | 123 | TS | 1 | CELLLN | Cell Line | |
|
This example Trial Sets dataset shows information about the test conditions for set A1 and A2 in this example study. Sets A1 and A2 can be seen in the first and second rows respectively of the sample report Table 1 (above). For brevity, the TX dataset and the findings (GT) dataset do not show information for any other sets. Fully formed datasets for this example study would include information about the test conditions and findings for all sets. | Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-457 |
|---|
|
This example Trial Sets dataset shows information about the test conditions for set A1 and A2 in this example study. Sets A1 and A2 can be seen in the first and second rows respectively of the sample report Table 1 (above). For brevity, the TX dataset and the findings (GT) dataset do not show information for any other sets. Fully formed datasets for this example study would include information about the test conditions and findings for all sets.
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Rows 1-23: | Show trial set parameters and values that comprise the test conditions for trial set A1. Set A1 is the data for the negative control (concentration 0) with short-term exposure and metabolic activation S9. The applicant has chosen to given a long name (SET) equal to "ST+S9_C0". Set A1 is associated with the first row in the sample report table for study 123. | | Rows 24-46: | Show trial set parameters and values that comprise the test conditions for trial set A2. Set A2 is the data for the short-term exposure with metabolic activation S9 at a concentration of 1250 ug/ml. The applicant has chosen to give the set a long name (SET) equal to "ST+S9_C1250". Set A2 is associated with the second row in the sample report table for study 123. |
|
| Dataset2 |
|---|
| Row | STUDYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|
| 1 | 123 | TX | A1 | ST+S9_C0 | 1 | MTACTIND | Metabolic Activating Agent Name | +S9 | | 2 | 123 | TX | A1 | ST+S9_C0 | 2 | METACTFL | Presence of Metabolic Activation Flag | Y | | 3 | 123 | TX | A1 | ST+S9_C0 | 3 | IVTDMIN | In vitro Treatment Duration Minimum | 3 | | 4 | 123 | TX | A1 | ST+S9_C0 | 4 | IVTDTRG | In vitro Treatment Duration Target | 3.5 | | 5 | 123 | TX | A1 | ST+S9_C0 | 5 | IVTDMAX | In vitro Treatment Duration Maximum | 4 | | 6 | 123 | TX | A1 | ST+S9_C0 | 6 | IVTDU | In vitro Treatment Duration Unit | HOURS | | 7 | 123 | TX | A1 | ST+S9_C0 | 7 | RCVDMIN | Recovery Duration Minimum | 23.5 | | 8 | 123 | TX | A1 | ST+S9_C0 | 8 | RCVDTRG | Recovery Duration Target | 24 | | 9 | 123 | TX | A1 | ST+S9_C0 | 9 | RCVDMAX | Recovery Duration Maximum | 24.5 | | 10 | 123 | TX | A1 | ST+S9_C0 | 10 | RCVDU | Recovery Duration Unit | HOURS | | 11 | 123 | TX | A1 | ST+S9_C0 | 11 | INCBTMP | Incubation Temperature | 37 | | 12 | 123 | TX | A1 | ST+S9_C0 | 12 | INCBTMPU | Incubation Temperature Unit | C | | 13 | 123 | TX | A1 | ST+S9_C0 | 13 | ATMRHP | Atmospheric Relative Humidity Percent | 50 | | 14 | 123 | TX | A1 | ST+S9_C0 | 14 | ATMCO2P | Atmospheric CO2 Percent | 5 | | 15 | 123 | TX | A1 | ST+S9_C0 | 15 | SPTOBID | Applicant-defined tobacco identifier | CIG01a | | 16 | 123 | TX | A1 | ST+S9_C0 | 16 | EXPTYP | | Submerged | | 17 | 123 | TX | A1 | ST+S9_C0 | 17 | SAMTYP | Sample Type | Total Particulate Matter in DMSO | | 18 | 123 | TX | A1 | ST+S9_CO | 18 | ITVNAM | Intervention Article Name | Tobacco ProdA | | 19 | 123 | TX | A1 | ST+S9_C0 | 19 | ITVTYPE | Intervention Article Type | Negative Control | | 20 | 123 | TX | A1 | ST+S9_C0 | 20 | ITVCONC | Intervention Article Concentration | 0 | | 21 | 123 | TX | A1 | ST+S9_C0 | 21 | ITVCONCU | Intervention Article Concentration Unit | ug/ml | | 22 | 123 | TX | A1 | ST+S9_C0 | 22 | SPDEVID | Applicant-defined device identifier | PUFFMASTER3K | | 23 | 123 | TX | A1 | ST+S9_C0 | 23 | SMKRGM | Smoking Regimen | MEDIUM INTENSITY REGIMEN | | 24 | 123 | TX | A2 | ST+S9_C1250 | 24 | MTACTIND | Metabolic Activating Agent Name | +S9 | | 25 | 123 | TX | A2 | ST+S9_C1250 | 25 | METACTFL | Presence of Metabolic Activation Flag | Y | | 26 | 123 | TX | A2 | ST+S9_C1250 | 26 | IVTDMIN | In vitro Treatment Duration Minimum | 3 | | 27 | 123 | TX | A2 | ST+S9_C1250 | 27 | IVTDTRG | In vitro Treatment Duration Target | 3.5 | | 28 | 123 | TX | A2 | ST+S9_C1250 | 28 | IVTDMAX | In vitro Treatment Duration Maximum | 4 | | 29 | 123 | TX | A2 | ST+S9_C1250 | 29 | IVTDU | In vitro Treatment Duration Unit | HOURS | | 30 | 123 | TX | A2 | ST+S9_C1250 | 30 | RCVDMIN | Recovery Duration Minimum | 23.5 | | 31 | 123 | TX | A2 | ST+S9_C1250 | 31 | RCVDTRG | Recovery Duration Target | 24 | | 32 | 123 | TX | A2 | ST+S9_C1250 | 32 | RCVDMAX | Recovery Duration Maximum | 24.5 | | 33 | 123 | TX | A2 | ST+S9_C1250 | 33 | RCVDU | Recovery Duration Unit | HOURS | | 34 | 123 | TX | A2 | ST+S9_C1250 | 34 | INCBTMP | Incubation Temperature | 37 | | 35 | 123 | TX | A2 | ST+S9_C1250 | 35 | INCBTMPU | Incubation Temperature Unit | C | | 36 | 123 | TX | A2 | ST+S9_C1250 | 36 | ATMRHP | Atmospheric Relative Humidity Percent | 50 | | 37 | 123 | TX | A2 | ST+S9_C1250 | 37 | ATMCO2P | Atmospheric CO2 Percent | 5 | | 38 | 123 | TX | A2 | ST+S9_C1250 | 38 | SPTOBID | Applicant-defined tobacco identifier | CIG01a | | 39 | 123 | TX | A2 | ST+S9_C1250 | 39 | EXPTYP | | Submerged | | 40 |
|
|
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Rows 1-17: | Show the experimental parameters that are common for table 1 of study 123. | | Rows 18, 25: | Show that sets A1 and A2 each have a parent set code of A. As a child of set A, each of these sets inherit the test conditions of set A. | | Rows 18-24: | Show trial set parameters and values that comprise the test conditions for trial set A1. Set A1 is the data for the negative control (concentration 0) with short-term exposure and metabolic activation S9. The applicant has chosen to given a long name (SET) equal to "ST+S9_C0". Set A1 is associated with the first row in the sample report table for study 123. | | Rows 25-31: | Show trial set parameters and values that comprise the test conditions for trial set A2. Set A2 is the data for the short-term exposure with metabolic activation S9 at a concentration of 1250 ug/ml. The applicant has chosen to give the set a long name (SET) equal to "ST+S9_C1250". Set A2 is associated with the second row in the sample report table for study 123. | | Dataset2 |
|---|
| Row | STUDYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|
| 123 | TX | A | ST+S9 | | METACTIND | Metabolic Activating Agent Name | +S9 | | 2 | 123 | TX | A | ST+S9 | 2 | METACTFL | Presence of Metabolic Activation Flag | Y | | 3 | 123 | TX | A | ST+S9 | 3 | IVTDMIN | In vitro Treatment Duration Minimum | 3 | | 4 | 123 | TX | A | ST+S9 | 4 | IVTDTRG | In vitro Treatment Duration Target | 3.5 | | 5 | 123 | TX | A | ST+S9 | 5 | IVTDMAX | In vitro Treatment Duration Maximum | 4 | | 6 | 123 | TX | A | ST+S9 | 6 | IVTDU | In vitro Treatment Duration Unit | HOURS | | 7 | 123 | TX | A | ST+S9 | 7 | RCVDMIN | Recovery Duration Minimum | 23.5 | | 8 | 123 | TX | A | ST+S9 | 8 | RCVDTRG | Recovery Duration Target | 24 | | 9 | 123 | TX | A | ST+S9 | 9 | RCVDMAX | Recovery Duration Maximum | 24.5 | | 10 | 123 | TX | A | ST+S9 | 10 | RCVDU | Recovery Duration Unit | HOURS | | 11 | 123 | TX | A | ST+S9 | 11 | INCBTMP | Incubation Temperature | 37 | | 12 | 123 | TX | A | ST+S9 | 12 | INCBTMPU | Incubation Temperature Unit | C | | 13 | 123 | TX | A | ST+S9 | 13 | ATMRHP | Atmospheric Relative Humidity Percent | 50 | | 14 | 123 | TX | A | ST+S9 | 14 | ATMCO2P | Atmospheric CO2 Percent | 5 | | 15 | 123 | TX | A | ST+S9 | 15 | SPTOBID | Applicant-defined tobacco identifier | CIG01a | | 16 | 123 | TX | A | ST+S9 | 16 | EXPTYP | | Submerged | | 17 | 123 | TX | A | ST+S9 | 17 | SAMTYP | Sample Type | Total Particulate Matter in DMSO | | 18 | 123 | TX | A1 | ST+S9_C0 | | PSETCD | Parent Set Code | A | | 19 | 123 | TX | A1 | ST+S9_CO | 2 | ITVNAM | Intervention Article Name | Tobacco ProdA | | 20 | 123 | TX | A1 | ST+S9_C0 | 3 | ITVTYPE | Intervention Article Type | Negative Control | | 21 | 123 | TX | A1 | ST+S9_C0 | 4 | ITVCONC | Intervention Article Concentration | 0 | | 22 | 123 | TX | A1 | ST+S9_C0 | 5 | ITVCONCU | Intervention Article Concentration Unit | ug/ml | | 23 | 123 | TX | A1 | ST+S9_C0 | 6 | SPDEVID | Applicant-defined device identifier | PUFFMASTER3K | | 24 | 123 | TX | A1 | ST+S9_C0 | 7 | SMKRGM | Smoking Regimen | MEDIUM INTENSITY REGIMEN | 251PSETCD | Parent Set Code | A | 26| SAMTYP | Sample Type | Total Particulate Matter in DMSO | | 41 | 123 | TX | A2 | ST+S9_C1250 |
2| 41 | ITVNAM | Intervention Article Name | Tobacco ProdA |
273| 42 | ITVTYPE | Intervention Article Type | Product |
284| 43 | ITVCONC | Intervention Article Concentration | 1250 |
295| 44 | ITVCONCU | Intervention Article Concentration Unit | ug/ml |
306| 45 | SPDEVID | Applicant-defined Device Identifier | PUFFMASTER2023 |
317| 46 | SMKRGM | Smoking Regimen | HIGH INTENSITY REGIMEN |
|
|
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Row 1: | Shows the value of REFID=C0. This REFID refers to the trial set with a SETCD of "A1", as defined in the TX dataset. LEVEL=1 and LVLDESC="EXPERIMENTAL UNIT/TRIAL SET" indicates this identifier is referring to both the experimental unit and the unit to which the treatment is applied, and to the entire trial set. | | Rows 2-5: | Show the values of 4 observational units (C0_Count1 through C0_Count4) that are within the parent experimental unit, REFID=C0. In this example assay, these observational units are also all within the same trial set, as defined in the TX dataset. | | Row 6: | Shows the value of REFID=C1250. This REFID refers to the trial set with a SETCD of "A2", as defined in the TX dataset. LEVEL=1 and LVLDESC="EXPERIMENTAL UNIT/TRIAL SET" indicates this identifier is referring to both the experimental unit and the unit to which the treatment is applied, and to the entire trial set. | | Rows 7-10: | Show the values of 4 observational units (C1250_Count1 through C1250_Count4) | Jira |
|---|
| showSummary | false |
|---|
server | Issue Tracker (JIRA) | serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-687 |
|---|
that are within the parent experimental unit, REFID=C1250. In this example assay, these observational units are also all within the same trial set, as defined in the TX dataset. |
|
| Dataset2 |
|---|
| Row | STUDYID | SETCD | REFID | PARENT | LEVEL | LVLDESC |
|---|
1 | 123 | | C0 |
| 1 | EXPERIMENTAL UNIT/TRIAL SET | 2 | 123 | A1 | C0-Count1 | C0 | 2 | OBSERVATIONAL UNIT | 3 | 123 | A1 | C0-Count2 | C0 | 2 | OBSERVATIONAL UNIT | 4 | 123 | A1 | C0-Count3 | C0 | 2 | OBSERVATIONAL UNIT | 5 | 123 | A1 | C0-Count4 | C0 | 2 | OBSERVATIONAL UNIT | 6 | 123 | A2 | C1250 |
| 1 | EXPERIMENTAL UNIT/TRIAL SET | | 7 | 123 | A2 | C1250-Count1 | C1250 | 2 | OBSERVATIONAL UNIT | | 8 | 123 | A2 | C1250-Count2 | C1250 | 2 | OBSERVATIONAL UNIT | | 9 | 123 | A2 | C1250-Count3 | C1250 | 2 | OBSERVATIONAL UNIT | | 10 | 123 | A2 | C1250-Count4 | C1250 | 2 | OBSERVATIONAL UNIT |
|
|
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
Rows 1-3, 8: | Show percentage result values that apply to GTREFID=C0. REFID=C0, as shown in the RELREF dataset, relates this data to the trial set in the first row of table 1 in the sample report table for study 123. | | Rows 4-7: | Show the 4 micronucleated cell counts for the observational units with GTREFID from C0-Count1 through C0-Count4, for which their relationship to test conditions (in tx.xpt) and experimental units (in relref.xpt) are shown in the RELREF dataset. | Rows 9-11, 16: | Show percentage result values that apply to GTREFID=C1250. REFID=C1250, as shown in the RELREF dataset, relates this data to the trial set in the second row of table 1 in the sample report table for study 123. | | Rows 12-15: | Show the 4 micronucleated cell counts for the observational units with GTREFID from C1250-Count1 through C1250-Count4, for which their relationship to test conditions (in tx.xpt) and experimental units (in relref.xpt) are shown in the RELREF dataset. |
|
| Dataset2 |
|---|
| Row | STUDYID | DOMAIN | GTSEQ | GTREFID |
|---|
GTSEQ | GTTESTCD | GTTEST | GTCELLEV | GTORRES | GTORRESU | GTCOLSRT | GTSTRESC | GTSTRESN | GTSTRESU | GTDTC |
|---|
| 1 |
123 | GT | C0 | 1 | RICC| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
key | TOBA-643 | Relative Increase in Cell Count | 154 | 0 | % |
| 0 | 0 | % | 2022-05-25 | | 2 | 123 | GT | 2 | C0 |
2 | | RCC | Relative Cell Count | 154 | 0 | % |
| 0 | 0 | % | 2022-05-25 | | 3 | 123 | GT | 3 | C0 |
3 | | RPD | Relative Population Doubling | 154 | 0 | % |
| 0 | 0 | % | 2022-05-25 | | 4 | 123 | GT | 4 | C0-Count1 |
4 | MNCE | Micronucleated Cells | 2205 | 15 |
CellsCells |
| 2022-05-25 | | 5 | 123 | GT | 5 | C0-Count2 |
5 | | MNCE | Micronucleated Cells | 2474 | 13 |
Cells | Cells |
| 2022-05-25 | | 6 | 123 | GT | 6 | C0-Count3 |
6 | MNCE | Micronucleated Cells | 2758 | 17 |
CellsCellsGT7 | MNCE | Micronucleated Cells | 2669 | 12 |
CellsCells8 | MNCECE | Micronucleated Cells/Total Cells |
| 0.57 | % |
| 0.57 | 0.57 | % | 2022-05-25 | | 9 | 123 | GT | 1 | C1250 |
1 | RICC | Relative Increase in Cell Count | 134 | 15.7 | % |
| 15.7 | 15.7 | % | 2022-05-25 | | 10 | 123 | GT | 2 | C1250 |
2 | RCC | Relative Cell Count | 134 | 13.0 | % |
| 13.0 | 13.0 | % | 2022-05-25 | | 11 | 123 | GT | 3 | C1250 |
3 | RPD | Relative Population Doubling | 134 | 7.9 | % |
| 7.9 | 7.9 | % | 2022-05-25 | | 12 | 123 | GT | 4 | C1250-Count1 |
4 | MNCE | Micronucleated Cells | 3266 | 20 |
Cells | Cells |
| 2022-05-25 | | 13 | 123 | GT | 5 | C1250-Count2 |
5 | MNCE | Micronucleated Cells | 2190 | 17 |
CellsCells |
| 2022-05-25 | | 14 | 123 | GT | 6 | C1250-Count3 |
6 | | MNCE | Micronucleated Cells | 2758 | 13 |
Cells | Cells |
| 2022-05-25 | | 15 | 123 | GT | 7 | C1250-Count4 |
7 | | MNCE | Micronucleated Cells | 2714 | 21 |
CellsCells8 | MNCECE | Micronucleated Cells/Total Cells |
| 0.66 | % |
| 0.66 | 0.66 | % | 2022-05-25 |
|
|
