This example shows a sample report table, trial design, and results dataset for Study #123 study 123 for the determination of the in vitro genotoxicity potential of tobacco products using the in vitro Micronucleus Assay| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-332 |
|---|
|
. | Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-135 |
|---|
|
| Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-127 |
|---|
|
| Expand |
|---|
| title | Sample Report Table for Study 123 |
|---|
|
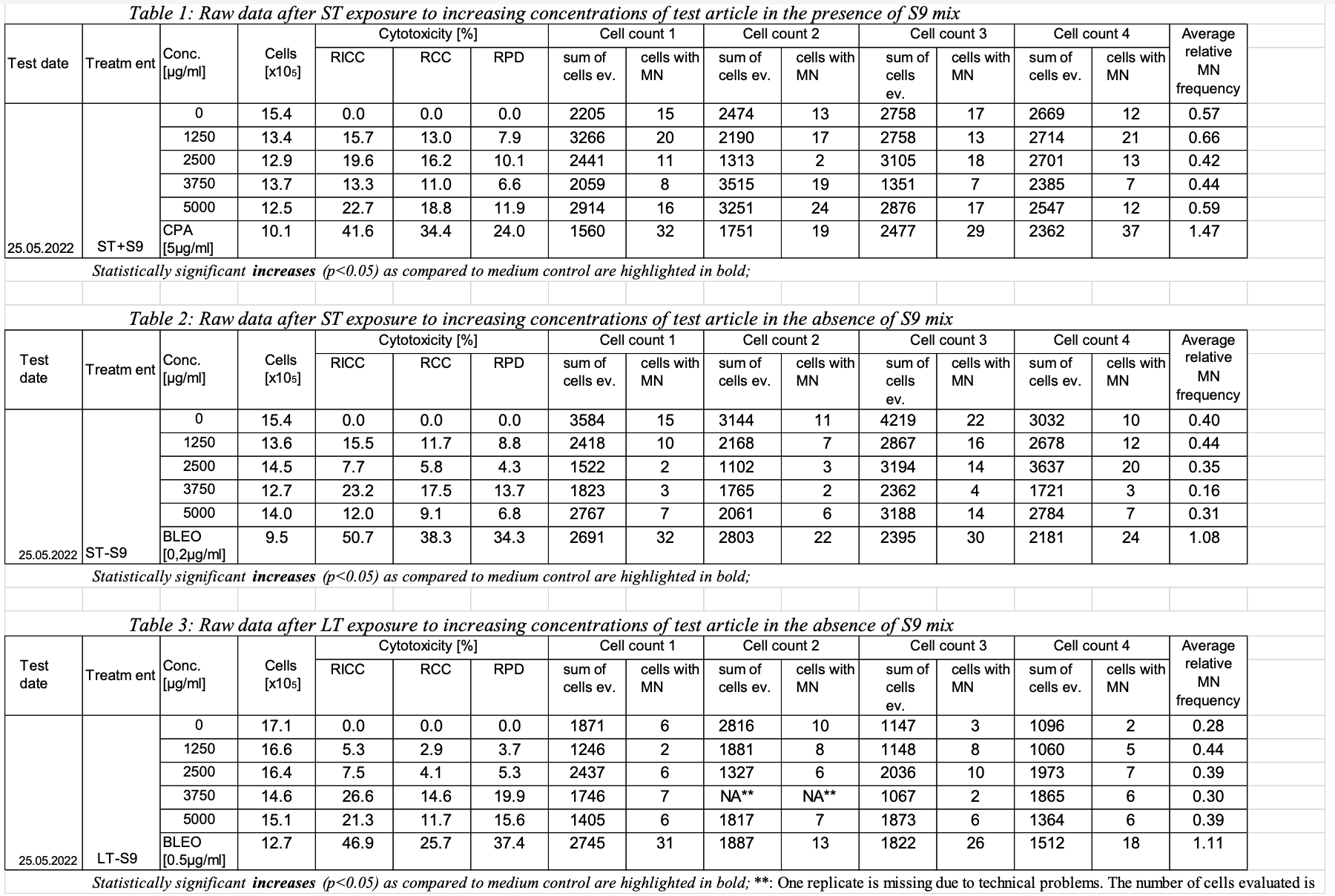 Image Removed Image Removed
|
micronucleus assay.
| Expand |
|---|
| title | Sample Report Table for Study 123 |
|---|
|
 Image Added Image Added
|
| Dataset wrap |
|---|
| Rowcaps |
|---|
| Rows 1-2: | Show 2 records for TSPARMCD = "GLPTYP", using TSSEQ to indicate multiple records, since both GLP types apply for this example study. | | Row 3: | Shows that this study was conducted as a GLP study. | | Rows 4-5: | Show the study start date and study title. | | Rows 6-7: | Show the version of SEND Implementation Guide and version of Controlled Terminology used in this study. | | Row 8: | Shows the applicant's organization. | | Row 9: | Shows that the applicant's study reference ID is not applicable. | | Rows 10-13: | Show that |
|
|
| Dataset wrap |
|---|
| Rowcaps |
|---|
| Rows 1-2: | Show two records for TSPARMCD = "GLPTYP", using TSSEQ to indicate multiple records, since both GLP Types apply for this example study. | | Row 8: | Shows that the sponsor's study reference ID is not applicable. | | Rows 9-12: | Show that TSGRPID has been used to link records (name, location, country) related to the test facility (TSGRPID = 1). The Study Director study director is associated with the Test Facility.test facility. | | Rows 14-16: | Show that TSGRPID (TSGRPID=2) has been used to link the information on the testing guideline followed on this study (TSTGDNAM, TSTGDORG, TSTGDVER). | | Shows the study type for this study. | | Shows that this study includes a Mammalian Cell Micronucleus Assay. | | Rows 19-20: | Show that the species is human and the cell line is TK6 lymphoblastoid in this study. |
|
| Dataset2 |
|---|
| Dataset2 |
|---|
Row | STUDYID | GNTXAID | DOMAIN | TSSEQ | TSGRPID | TSPARMCD | TSPARM | TSVAL | TSVALNF |
|---|
| 1 | 123 |
NRU | | TS | 1 |
| GLPTYP | Good Laboratory Practice Type | FDA |
| | 2 | 123 |
NRU | | TS | 2 |
| GLPTYP | Good Laboratory Practice Type | OECD |
| | 3 | 123 |
NRUSTSTDTC | Study Start Date | 2022
| GLPFL | GLP Flag | Y |
| | 4 | 123 | TS | 1 |
| STSTDTC | Study Start Date | 2022-05-25 |
4NRU | TS | 1 |
| STITLE | Study Title | Determination of the in vitro genotoxicity potential using the in vitro Neutral Red Uptake assay |
5NRU | TS | 1 |
| SNDIGVER | SEND Implementation Guide Version | TOBACCO IMPLEMENTATION GUIDE VERSION 1.0 |
6NRU | | TS | 1 |
| SNDCTVER | SEND Controlled Terminology Version | SEND Terminology 2021-09-30 |
7NRUSSPONSORSponsor Organization Sponsor 8NRU | SPREFIDSponsor | Study Reference ID |
| NOT APPLICABLE |
9NRU | | TS | 1 | 1 | TSTFNAM | Test Facility Name | Example Test Lab Name |
10NRU | TS | 1 | 1 | TSTFLOC | Test Facility Location | 10 Somewhere Street, Montgomery, AL 10000 |
11NRU | | TS | 1 | 1 | TFCNTRY | Test Facility Country | USA |
12NRU | TS | 1 | 1 | STDIR | Study Director | Dr. R. Smith |
13NRUGLPFLOECD GLP Flag | Y | 14 | 123 | NRU | TS | 1 | ASTD | Assay Standard | | TSTGDNAM | Testing Guideline Name |
| GUIDELINE FOR THE TESTING OF CHEMICALS No. 487 |
| | 15 | 123 |
NRUASTDV | Assay Standard Version | | TSTGDORG | Testing Guideline Organization | OECD |
29-July-2016NRUSSTYPStudy Type | GENOTOXICITY IN VITRO| TSTGDVER | Testing Guideline Version | 29-July-2016 |
| | 17 | 123 |
NRU | SSSTYP Sub MICRONUCLEUS TEST| GENOTOXICITY IN VITRO |
| | 18 | 123 |
| TS | 1 |
| GNTXAID | Genetic Toxicology Assay Identifier | MNvit |
| | 19 | 123 |
NRU19NRU | |
|
This example trial set dataset, tx.xpt, Trial Sets dataset shows information about the test conditions for set A1 and A2 in this example study, 123. Set Sets A1 and A2 can be seen in the first and second rows respectively of Table 1 in the Sample Report table for Study 123 the sample report Table 1 (above). For brevity, the trial sets dataset, tx.xpt, TX dataset and the findings dataset, gt.xpt (GT) dataset do not show information for any other sets. Fully formed datasets for this example study would include information about the test conditions and findings for all sets.
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
17 the experimental that are common for Table 1 of Study 123.and values that comprise the test conditions for trial set A1. Set A1 is the data for the negative control (concentration 0) with short-term exposure and metabolic activation S9. The applicant has chosen to given a long name (SET) equal to "ST+S9_C0". Set A1 is associated with the first row in the sample report table for study 123. | | Rows 24-46 |
| Rows 18, 25: | Show that set A1 and A2 each have a parent set code of A. As a child of set A, each of these sets inherit the test conditions of set A. | Rows 18-24| : | Show trial set parameters and |
trial set values that comprise the test conditions for trial set |
A1 A1 negative control (concentration 0) with and with metabolic activation S9 |
. at a concentration of 1250 ug/ml. The applicant has chosen to |
given give the set a long name (SET) equal to "ST+S9_ |
C0 A1 A2 is associated with the |
first second row in the sample report table for |
Study | Rows 25-31: | Show trial set parameters and trial set values that comprise the test conditions for trial set A2. Set A2 is the data for the short term exposure with metabolic activation S9 at a concentration of 1250 ug/ml. The applicant has chosen to give the set a long name (SET) equal to "ST+S9_C1250". Set A2 is associated with the second row in the sample report table for Study 123. | |
| Dataset2 |
|---|
| Row | STUDYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|
| 1 | 123 | TX | A1 | ST+S9_C0 | 1 | MTACTIND | Metabolic Activating Agent Name | +S9 | | 2 | 123 | TX | A1 | ST+S9_C0 | 2 | METACTFL | Presence of Metabolic Activation Flag | Y | | 3 | 123 | TX | A1 | ST+S9_C0 | 3 | IVTDMIN | In vitro Treatment Duration Minimum | 3 | | 4 | 123 | TX | A1 | ST+S9_C0 | 4 | IVTDTRG | In vitro Treatment Duration Target | 3.5 | | 5 | 123 | TX | A1 | ST+S9_C0 | 5 | IVTDMAX | In vitro Treatment Duration Maximum | 4 | | 6 | 123 | TX | A1 | ST+S9_C0 | 6 | IVTDU | In vitro Treatment Duration Unit | HOURS | | 7 | 123 | TX | A1 | ST+S9_C0 | 7 | RCVDMIN | Recovery Duration Minimum | 23.5 | | 8 | 123 | TX | A1 | ST+S9_C0 | 8 | RCVDTRG | Recovery Duration Target | 24 | | 9 | 123 | TX | A1 | ST+S9_C0 | 9 | RCVDMAX | Recovery Duration Maximum | 24.5 | | 10 | 123 | TX | A1 | ST+S9_C0 | 10 | RCVDU | Recovery Duration Unit | HOURS | | 11 | 123 | TX | A1 | ST+S9_C0 | 11 | INCBTMP | Incubation Temperature | 37 | | 12 | 123 | TX | A1 | ST+S9_C0 | 12 | INCBTMPU | Incubation Temperature Unit | C | | 13 | 123 | TX | A1 | ST+S9_C0 | 13 | ATMRHP | Atmospheric Relative Humidity Percent | 50 | | 14 | 123 | TX | A1 | ST+S9_C0 | 14 | ATMCO2P | Atmospheric CO2 Percent | 5 | | 15 | 123 | TX | A1 | ST+S9_C0 | 15 | SPTOBID | Applicant-defined tobacco identifier | CIG01a | | 16 | 123 | TX | A1 | ST+S9_C0 | 16 | EXPTYP | | Submerged | | 17 | 123 | TX | A1 | ST+S9_C0 | 17 | SAMTYP | Sample Type | Total Particulate Matter in DMSO | | 18 | 123 | TX | A1 | ST+S9_CO | 18 | ITVNAM | Intervention Article Name | Tobacco ProdA | | 19 | 123 | TX | A1 | ST+S9_C0 | 19 | ITVTYPE | Intervention Article Type | Negative Control | | 20 | 123 | TX | A1 | ST+S9_C0 | 20 | ITVCONC | Intervention Article Concentration | 0 | | 21 | 123 | TX | A1 | ST+S9_C0 | 21 | ITVCONCU | Intervention Article Concentration Unit | ug/ml | | 22 | 123 | TX | A1 | ST+S9_C0 | 22 | SPDEVID | Applicant-defined device identifier | PUFFMASTER3K | | 23 | 123 | TX | A1 | ST+S9_C0 | 23 | SMKRGM | Smoking Regimen | MEDIUM INTENSITY REGIMEN | | 24 | 123 | TX | A2 | ST+S9_C1250 | 24 | MTACTIND | Metabolic Activating Agent Name | +S9 | | 25 | 123 | TX | A2 | ST+S9_C1250 | 25 | METACTFL | Presence of Metabolic Activation Flag | Y | | 26 | 123 | TX | A2 | ST+S9_C1250 | 26 | IVTDMIN | In vitro Treatment Duration Minimum | 3 | | 27 | 123 | TX | A2 | ST+S9_C1250 | 27 | IVTDTRG | In vitro Treatment Duration Target | 3.5 | | 28 | 123 | TX | A2 | ST+S9_C1250 | 28 | IVTDMAX | In vitro Treatment Duration Maximum | 4 | | 29 | 123 | TX | A2 | ST+S9_C1250 | 29 | IVTDU | In vitro Treatment Duration Unit | HOURS | | 30 | 123 | TX | A2 | ST+S9_C1250 | 30 | RCVDMIN | Recovery Duration Minimum | 23.5 | | 31 | 123 | TX | A2 | ST+S9_C1250 | 31 | RCVDTRG | Recovery Duration Target | 24 | | 32 | 123 | TX | A2 | ST+S9_C1250 | 32 | RCVDMAX | Recovery Duration Maximum | 24.5 | | 33 | 123 | TX | A2 | ST+S9_C1250 | 33 | RCVDU | Recovery Duration Unit | HOURS | | 34 | 123 | TX | A2 | ST+S9_C1250 | 34 | INCBTMP | Incubation Temperature | 37 | | 35 | 123 | TX | A2 | ST+S9_C1250 | 35 | INCBTMPU | Incubation Temperature Unit | C | | 36 | 123 | TX | A2 | ST+S9_C1250 | 36 | ATMRHP | Atmospheric Relative Humidity Percent | 50 | | 37 | 123 | TX | A2 | ST+S9_C1250 | 37 | ATMCO2P | Atmospheric CO2 Percent | 5 | | 38 | 123 | TX | A2 | ST+S9_C1250 | 38 | SPTOBID | Applicant-defined tobacco identifier | CIG01a | | 39 | 123 | TX | A2 | ST+S9_C1250 | 39 | EXPTYP | | Submerged | | 40 | 123 |
| | Dataset2 |
|---|
| Row | STUDYID | GNTXAID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|
| 123 | MNvit | TX | A | ST+S9 | | METACT | Metabolic Activation | +S9 | | 2 | 123 | MNvit | TX | A | ST+S9 | 2 | METACTFL | Y/N presence of metabolic activation | Y | | 3 | 123 | MNvit | TX | A | ST+S9 | 3 | TRTDMIN | Treatment Duration Minimum | 3 | | 4 | 123 | MNvit | TX | A | ST+S9 | 4 | TRTDTRG | Treatment Duration Target | 3.5 | | 5 | 123 | MNvit | TX | A | ST+S9 | 5 | TRTDMAX | Treatment Duration Maximum | 4 | | 6 | 123 | MNvit | TX | A | ST+S9 | 6 | TRTDU | Treatment Duration Unit | HOURS | | 7 | 123 | MNvit | TX | A | ST+S9 | 7 | RCVDMIN | Recovery Duration Minimum | 23.5 | | 8 | 123 | MNvit | TX | A | ST+S9 | 8 | RCVDTRG | Recovery Duration Target | 24 | | 9 | 123 | MNvit | TX | A | ST+S9 | 9 | RCVDMAX | Recovery Duration Maximum | 24.5 | | 10 | 123 | MNvit | TX | A | ST+S9 | 10 | RCVDU | Recovery Duration Unit | HOURS | | 11 | 123 | MNvit | TX | A | ST+S9 | 11 | INCBTMP | Incubation Temperature | 37 | | 12 | 123 | MNvit | TX | A | ST+S9 | 12 | INCBTMPU | Incubation Temperature Unit | C | | 13 | 123 | MNvit | TX | A | ST+S9 | 13 | HUMID | Atmospheric Relative Humidity Percent | 50 | | 14 | 123 | MNvit | TX | A | ST+S9 | 14 | ATMCO2 | Atmospheric CO2 Percent | 5 | | 15 | 123 | MNvit | TX | A | ST+S9 | 15 | SPTOBID | Sponsor defined tobacco identifier | CIG01a | | 16 | 123 | MNvit | TX | A | ST+S9 | 16 | EXPTYP | | Submerged | | 17 | 123 | MNvit | TX | A | ST+S9 | 17 | SAMTYP | Sample Type | Total Particulate Matter in DMSO | | 18 | 123 | MNvit | TX | A1 | ST+S9_C0 | | PSETCD | Parent Set Code | A | | 19 | 123 | MNvit | TX | A1 | ST+S9_CO | 2 | INTRVN | Name of the Intervention Article | Tobacco ProdA | | 20 | 123 | MNvit | TX | A1 | ST+S9_C0 | 3 | ITVTYPE | type of intervention article | Negative Control | | 21 | 123 | MNvit | TX | A1 | ST+S9_C0 | 4 | ITVCONC | Concentration of intervention article | 0 | | 22 | 123 | MNvit | TX | A1 | ST+S9_C0 | 5 | ITVCONCU | Concentration Unit | ug/ml | | 23 | 123 | MNvit | TX | A1 | ST+S9_C0 | 6 | SPDEVID | Sponsor defined device identifier | PUFFMASTER3K | | 24 | 123 | MNvit | TX | A1 | ST+S9_C0 | 7 | DUREFID | Smoke Regimen | Medium Intensity Regimen | 25 | 123 | MNvit1PSETCD | Parent Set Code | A | 26 | 123 | | SAMTYP | Sample Type | Total Particulate Matter in DMSO | | 41 | 123 |
MNvitName of the 2 | INTRVN | Intervention Article Name | Tobacco ProdA |
27MNvit3type of intervention articleIntervention Article Type | Product |
28MNvit | 4| 43 | ITVCONC | Intervention Article Concentration |
of intervention article29MNvit5| 44 | ITVCONCU | Intervention Article Concentration Unit | ug/ml |
30MNvit | 6Sponsor defined device identifier| Applicant-defined Device Identifier | PUFFMASTER2023 |
31MNvit7DUREFIDSmoke High Intensity Regimen |
|
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Row 1: | Shows the value of REFID=C0. This REFID refers to the trial set with a SETCD of '"A1'", as defined in the trial set TX dataset, tx. xpt. LEVEL=1 and LVLDESC='"EXPERIMENTAL UNIT/TRIAL SET' " indicates this identifier is referring to both the experimental unit , and the unit to which the treatment is applied, and to the entire trial set. | | Rows 2-5: | Show the values of four 4 observational units (C0_Count1 through C0_Count4) that are within the parent experimental unit, REFID=C0, and in . In this example assay, these observational units are also all within the same trial set, as defined in the trial set TX dataset, tx.xpt. | | Row 6: | Shows the value of REFID=C1250. This REFID refers to the trial set with a SETCD of '"A2'", as defined in the trial set dataset, tx.xpt. TX dataset. LEVEL=1 and LVLDESC='"EXPERIMENTAL UNIT/TRIAL SET' " indicates this identifier is referring to both the experimental unit , and the unit to which the treatment is applied, and to the entire trial set. | | Rows 7-10: | four 4 observational units (C1250_Count1 through C1250_Count4) that are within the parent experimental unit, REFID= C1250. In this example assay, these observational units are also all within the same trial set, as defined in the |
|
| Dataset2 |
|---|
| GNTXAID | SETCD | REFID | PARENT | LEVEL | LVLDESC |
|---|
1 | 123 |
MNvit | | C0 |
| 1 | EXPERIMENTAL UNIT/TRIAL SET | 2 | 123 |
MNvit | A1 | C0-Count1 | C0 | 2 | OBSERVATIONAL UNIT | 3 | 123 |
MNvit | A1 | C0-Count2 | C0 | 2 | OBSERVATIONAL UNIT | 4 | 123 |
MNvit | A1 | C0-Count3 | C0 | 2 | OBSERVATIONAL UNIT | 5 | 123 |
MNvit | A1 | C0-Count4 | C0 | 2 | OBSERVATIONAL UNIT | 6 | 123 |
MNvit | A2 | C1250 |
| 1 | EXPERIMENTAL UNIT/TRIAL SET | | 7 | 123 |
MNvit | | A2 | C1250-Count1 | C1250 | 2 | OBSERVATIONAL UNIT | | 8 | 123 |
MNvit | A2 | C1250-Count2 | C1250 | 2 | OBSERVATIONAL UNIT | | 9 | 123 |
MNvit | | A2 | C1250-Count3 | C1250 | 2 | OBSERVATIONAL UNIT | | 10 | 123 |
MNvit | | A2 | C1250-Count4 | C1250 | 2 | OBSERVATIONAL UNIT |
|
|
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
Rows 1-3, 8: | Show percentage result values that apply to GTREFID=C0. REFID=C0, as shown in the relref RELREF dataset, relates this data to the trial set in the first row of Table table 1 in the sample report table for study 123. | | Rows 4-7: | Show the four 4 micronucleated cell counts for the observational units with GTREFID from C0-Count1 through C0-Count4, for which their relationship to test conditions (in tx.xpt) and experimental units (in relref.xpt) are shown in the relref RELREF dataset. | Rows 9-11, 16: | Show percentage result values that apply to GTREFID=C1250. REFID=C1250, as shown in the relref RELREF dataset, relates this data to the trial set in the second row of Table table 1 in the sample report table for study 123. | | Rows 12-15: | Show the four 4 micronucleated cell counts for the observational units with GTREFID from C1250-Count1 through C1250-Count4, for which their relationship to test conditions (in tx.xpt) and experimental units (in relref.xpt) are shown in the relref RELREF dataset. |
|
| Dataset2 |
|---|
GNTXAIDDOMAINGTSEQ | GTTESTCD | GTTEST | GTCELLEV | GTORRES | GTORRESU | GTCOLSRT | GTSTRESC | GTSTRESN | GTSTRESU | GTDTC |
|---|
| 1 | 123 |
MNvitGT1 | | Relative Increase in Cell Count | 154 | 0 | % |
| 0 | 0 | % | 2022-05-25 | | 2 | 123 |
MNvitGT2 | | RCC | Relative Cell Count | 154 | 0 | % |
| 0 | 0 | % | 2022-05-25 | | 3 | 123 |
MNvitGT3 | RPD | Relative Population Doubling | 154 | 0 | % |
| 0 | 0 | % | 2022-05-25 | | 4 | 123 |
MNvitGT4 | MNCELLS| MNCE | Micronucleated Cells | 2205 | 15 |
CellsCells | MNvitGT5MNCELLS | | Micronucleated Cells | 2474 | 13 |
CellsCells | MNvitGTMNCELLS6 | | MNCE | Micronucleated Cells | 2758 | 17 |
CellsCellsMNvitGTMNCELLS7 | | MNCE | Micronucleated Cells | 2669 | 12 |
Cells | Cells | MNvitGT8 | AVGREL | Average Relative MN Frequency| MNCECE | Micronucleated Cells/Total Cells |
| 0.57 | % |
| 0.57 | 0.57 | % | 2022-05-25 | | 9 | 123 |
MNvitGT1 | | RICC | Relative Increase in Cell Count | 134 | 15.7 | % |
| 15.7 | 15.7 | % | 2022-05-25 | | 10 | 123 |
MNvitGT2 | | RCC | Relative Cell Count | 134 | 13.0 | % |
| 13.0 | 13.0 | % | 2022-05-25 | | 11 | 123 |
MNvitGT3 | RPD | Relative Population Doubling | 134 | 7.9 | % |
| 7.9 | 7.9 | % | 2022-05-25 | | 12 | 123 |
MNvitGTMNCELLS4 | | MNCE | Micronucleated Cells | 3266 | 20 |
CellsCellsMNvitGT5MNCELLS | | Micronucleated Cells | 2190 | 17 |
Cells | Cells | MNvitGTMNCELLS6 | | MNCE | Micronucleated Cells | 2758 | 13 |
Cells | CellsMNvitGTMNCELLS7 | | MNCE | Micronucleated Cells | 2714 | 21 |
Cells | CellsMNvitGT8 | AVGREL | | MNCECE | Micronucleated Cells/Total Cells |
Average Relative MN Frequency |
|
