This is an example showing the report table, trial design, and results from the a study's in vitro bacterial reverse mutation test of an example study, Study #8325064. This bacterial reverse mutation test uses four using 4 different amino acid-requiring strains of Salmonella typhimurium (S. typhimurium) and one 1 strain of Escherichia coli (E. coli) to detect point mutations, which involve substitution, addition or deletion of one or a few DNA base pairs. jira
...
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-129 |
|---|
| title | Report Table for Example Study 8325064 |
|---|
|
This table shows the assay results for study 8325064, test article TA1, and 3 strains of salmonella (TA98, TA100, and TA1535) at varying concentrations. For brevity, the remaining tables (e.g., additional strains, samples prepared without metabolic activation) are not included. For ease of reference in the table below, each row has been labeled in the right-hand margin with the applicant-defined trial set label (e.g., SetA). For brevity, only the four bolded sets are represented in the following example datasets.
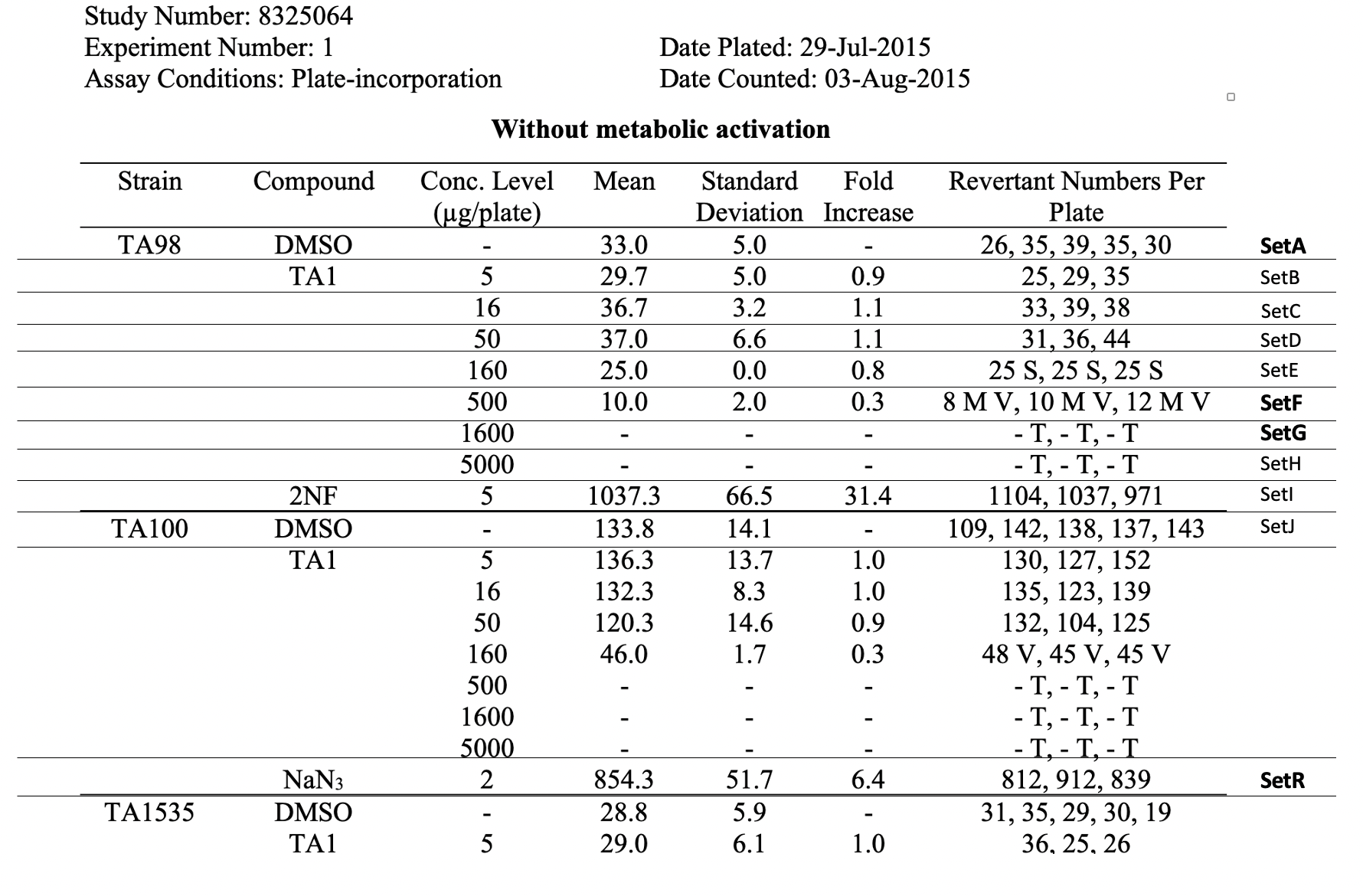 Image Added Image Added
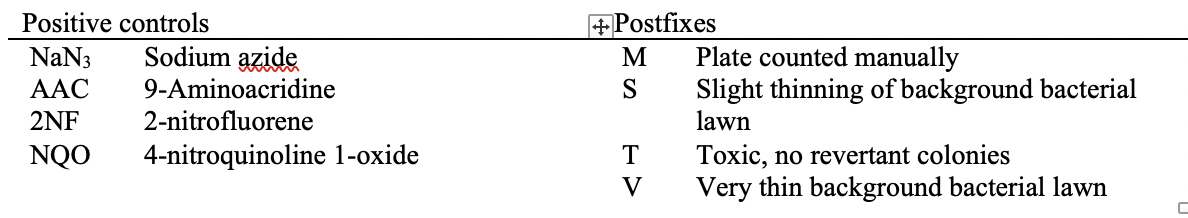 Image Added Image Added
|
This example Trial Summary (TS) dataset, shows many informational fields that provide context at the study level.
...
In this example, the Trial Summary dataset, ts.xpt, includes many informational fields that may provide context at the study level, for Study 8325064. Also, TSGRPID has been used to link records (name, location, country) related to the test facility (TSGRPID = 1) and records related to the Test Site (TSGRPID = 2). The Study Director is associated with the Test Facility and the Principal Investigator is associated with the Test Site. Finally, the Primary Treatment CAS Registry Number is not known and this is recorded as an empty TSVAL and UNKNOWN in the corresponding TSVALNF.
| Expand |
|---|
| title | Report Table for Example Study 8325064 |
|---|
|
This table shows the assay results for Study 8325064, Test Article TA1, and three strains of Salmonella (TA98, TA100, and TA1535) at varying concentrations. For ease of reference, each row has been labeled in the right hand margin with the sponsor defined trial set label (e.g., SetA). For brevity, the remaining tables (e.g., additional strains, samples prepared without metabolic activation, etc.) are not included.  Image Removed Image Removed
 Image Removed Image Removed
|
| Dataset wrap |
|---|
| Rowcaps |
|---|
| Rows 1-2: | two 2 records for TSPARMCD = "GLPTYP", using TSSEQ to indicate multiple records, | | Row 83: | Shows that the sponsor's study reference ID is not applicablethis study was conducted as a GLP study. | | Rows 94-125: | Show that TSGRPID has been used to link records (name, location, country) related to the test facility (TSGRPID = 1). The Study Director is associated with the Test Facility. | | Rows 18-24: | Show that this example study includes two different species of bacteria and a total of five strains. | | Rows 18-22: | Show that TSGRPID (TSGRPID = 2) has been used to link the information for one species (Salmonella) with four different strains that are tested in this example study. | | the study start date and study title. | | Rows 6-7: | Show the version of SEND Implementation Guide and version of Controlled Terminology used in this study. | | Row 8: | Shows the applicant's organization. | | Row 9: | Shows that the applicant's study reference ID is not applicable. | | Rows 10-13: | Show that TSGRPID has been used to link records (name, location, country) related to the test facility (TSGRPID=1). The study director is associated with the test facility. | | Rows 14-16Rows 23-24: | Show that TSGRPID (TSGRPID= 4) has been used to link the information on the testing guideline followed on this study (TSTGDNAM, TSTGDORG, TSTGDVER). | | Shows the study type for this study. | | Shows that this study includes a Bacterial Reverse Mutation Assay. | | Rows 19-27: | Show that TSGRPID (TSGRPID = 2) has been used to link the information for 1 species (salmonella) with the 4 different strains and cell lines that are tested in this study. | | Rows 28-30: | Show that TSGRPID (TSGRPID = 3) has been used to link the information for 1 species (E. coli) with the strain and cell line that is tested in this study. |
|
| Dataset2 |
|---|
| Dataset2 |
|---|
Row | STUDYID | GNTXAID | DOMAIN | TSSEQ | TSGRPID | TSPARMCD | TSPARM | TSVAL | TSVALNF |
|---|
1 | 8325064 | Ames | TS | 1 | GLPTYP | | Row | STUDYID | DOMAIN | TSSEQ | TSGRPID | TSPARMCD | TSPARM | TSVAL | TSVALNF |
|---|
| 1 | 8325064 | TS | 1 |
| GLPTYP |
| Good Laboratory Practice Type | FDA |
| | 2 | 8325064 |
Ames | TS | 2 |
| GLPTYP | Good Laboratory Practice Type | OECD |
| | 3 | 8325064 |
Ames| TS | 1 |
| GLPFL | GLP Flag | N |
| | 4 | 8325064 | TS | 1 |
| STSTDTC | Study Start Date | 2015-07-29 |
4Ames | | TS | 1 |
| STITLE | Study Title | The Bacterial Reverse Mutation Test, Study 8325064-1 |
5AmesTS | | 1 |
| SNDIGVER | SEND Implementation Guide Version | TOBACCO IMPLEMENTATION GUIDE VERSION 1.0 |
6Ames | TS | 1 |
| SNDCTVER | SEND Controlled Terminology Version | SEND Terminology 2021-09-30 |
7AmesSSPONSORSponsor Organization Sponsor 8AmesSPREFIDSponsor| Applicant's Study Reference ID |
| NOT APPLICABLE |
9Ames | TS | 1 | 1 | TSTFNAM | Test Facility Name | Example Tox Lab Name |
10Ames | | TS | 1 | 1 | TSTFLOC | Test Facility Location | 10 Somewhere Street, Montgomery, AL 10000 |
11Ames | | TS | 1 | 1 | TFCNTRY | Test Facility Country | USA |
12Ames | | TS | 1 | 1 | STDIR | Study Director | Dr. R. Smith |
13AmesGLPFL | GLP Flag | N | | 4 | TSTGDNAM | Testing Guideline Name | Test NO. 471 |
| | 15 |
14Ames | ASTD | Assay Standard| TSTGDORG | Testing Guideline Organization | OECD |
TEST NO. 47115Ames | ASTDV | | 4 | TSTGDVER | Testing Guideline |
Assay Standard 16Ames | | TS | 1 |
| SSTYP | Study Type | GENOTOXICITY IN VITRO |
17Ames | SSSTYP | Study Sub Type | BACTERIAL REVERSE MUTATION TEST | 18 | 8325064 |
| | Genetic Toxicology Assay Identifier | |
| | 19 | 8325064 |
Ames19 | 8325064 | Ames | | Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-123 |
|---|
| | TS | 1 | 2 | STRAIN | Strain/Substrain | TA98 |
20Ames | 1| 2 | 2 | STRAIN | Strain/Substrain | TA100 |
21Ames | 1| 3 | 2 | STRAIN | Strain/Substrain | TA1535 |
22Ames | 1| 4 | 2 | STRAIN | Strain/Substrain | TA1537 |
23Ames3SPECIESSpecies | Escherichia coli | 24 | 8325064 | Ames | TS | 1 | 3 | STRAIN | Strain/Substrain | WP2 uvrA pKM101 | |
|
This example trial set dataset, tx.xpt, shows information about the test conditions for SetA and SetR in this example study. For brevity, this does not show information for SetF, SetG, or any other sets. A fully formed tx.xpt for this example study would include information about the test conditions for all sets.
| Cell Line | TA 98 hisD3052; rfa-; uvrB- |
| | 25 | 8325064 | TS | 2 | 2 | CELLLN | Cell Line | TA 100 hisG46; rfa-; uvrB- |
| | 26 | 8325064 | TS | 3 | 2 | CELLLN | Cell Line | TA 1535 hisG46; rfa-; uvrB- |
| | 27 | 8325064 | TS | 4 | 2 | CELLLN | Cell Line | TA 1537 hisC3076; rfa-; uvrB- |
| | 28 | 8325064 | TS | 2 | 3 | SPECIES | Species | |
| | 29 | 8325064 | TS | 5 | 3 | STRAIN | Strain/Substrain | WP2 uvrA pKM101 |
| | 30 | 8325064 | TS | 5 | 3 | CELLLN | Cell Line | trpE uvrA |
|
|
|
This example Trial Set (TX) dataset shows information about the test conditions for SetA and SetR in this study. For brevity, the dataset does not show information for SetF, SetG, or any other sets. A fully formed TX dataset for this example study would include information about the test conditions for all sets.
Note that there are three trial set parameters that link to other important datasets: SPTOBID, APDEVID, and SMKRGM.
- SPTOBID (Applicant-Defined Tobacco Product ID) is used to uniquely identify the tobacco product. The value of SPTOBID (e.g., CIG01a) matches the value for SPTOBID in all the TOPARMCD-TOVAL pairs in the Tobacco Product Identifiers and Descriptors (TO) dataset example in Section 3.1.2, Product Design Parameters and Conformance Testing, Example 1. The TOPARMCD-TOVAL pairs identify this unique product, CIG01a. The TO domain is described in Section 2.8.8.1, Tobacco Product Identifiers and Descriptors (TO).
- The value of APDEVID (e.g., PUFFMASTER3K) matches the value of SPDEVID in all the DIPARMCD-DIVAL pairs that identify this unique device in the DI dataset. SPDEVID is the applicant-defined device identifier that is used to uniquely identify the device in the Unique Device Identification (DI) dataset (see also Section 2.8.9.7, SEND Device Identifiers (DI)). This is shown in the DI dataset in Section 3.1.3.2, HPHCs, Other Constituents, and Smoking/Vaping Regimens, Example 1.
- SMKRGM serves as a link to the Device-In Use Properties (DU) domain (see also Section 2.8.9.8, SEND Device-In-Use (DU)), where a matching value of SMKRGM indicates parameters of the smoking regimen performed by the smoking machine, as in the DU dataset in Section 3.1.3.2, HPHCs, Other Constituents, and Smoking/Vaping Regimens, Example 2.
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Rows 1-22: | Show trial set parameters and trial set values that are the test conditions specific to set SetA which is the vehicle control (i.e., Strain of TA98, vehicle control with concentration value of 0). SetA is associated with the first row, labeled "SetA", in the report table for example study 8325064. | | Rows 23-44: | Show trial set parameters and trial set values that comprise the test conditions for the set SetF which is the strain of TA98 with a concentration value of 500 µg/plate. SetF is associated with the sixth row, labeled "SetF", in the report table for example study 8325064. | | Rows 45-66: | Show trial set parameters and trial set values that comprise the test conditions for the set SetG which is the strain of TA98 with a concentration value of 1600 µg/plate. SetG is associated with the seventh row, labeled "SetG", in the report table for example study 8325064. | | Rows 67-88: | Show trial set parameters and trial set values that comprise the test conditions for the set SetR which is the strain of TA100, positive control with a concentration value of 2 µg/plate. SetR is associated with the eighteenth row, labeled "SetR", in the report table for example study 8325064. |
|
|
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Rows 1-22: | Show trial set parameters and trial set values that comprise the test conditions for the set SetA. SetA is the data for the vehicle control (i.e., Strain of TA98, vehicle control with concentration value of 0). SetA is associated with the first row, labeled "SetA", in the report table for example study 8325064. | | Rows 23-44: | Show trial set parameters and trial set values that comprise the test conditions for the set SetR. SetR is the data for the vehicle control (i.e., Strain of TA100, positive control with a concentration value of 2). SetR is associated with the eighteenth row, labeled "SetR", in the report table for example study 8325064. | | Rows 13, 35: | Show a value for SPTOBID=CIG01a. SPTOBID is Sponsor-Defined Tobacco Product ID that is used to uniquely identify the tobacco product. The value of SPTOBID, in this case CIG01a, matches the value for SPTOBID in all the TOPARMCD-TOVAL pairs in the Tobacco Product Identifiers and Descriptors (TO) dataset example in Section 3.1.2 Product Design Parameters and Conformance Testing Example 1. The TOPARMCD-TOVAL pairs identify this unique product, CIG01a. The TO domain is described in the Section 2.8.8.1 Tobacco Product Identifiers and Descriptors (TO). | | Rows 21, 43: | Show a value for SPDEVID. SPDEVID is the Sponsor defined device identifier that is used to uniquely identify the device in the Unique Device Identification (DI) dataset. The value of SPDEVID, in this case PUFFMASTER3K, matches the value of SPDEVID in all the DIPARMCD-DIVAL pairs that identify this unique device in the DI dataset. This is shown in the di.xpt in SDTM Example.Product HPHC ENDS Testing. The DI domain is described in the Section 2.8.9.7 Device Identifiers (DI). | | Rows 22, 44: | Show a value for DUREFID. DUREFID serves as a link to the Device-In Use Properties (DU) domain, where a matching value of DUREFID indicates parameters of the smoking regimen performed by the smoking machine, as in the du.xpt in SDTM Example.Product HPHC ENDS Testing. The DU domain is described in the Section 2.8.9.8 Device-In-Use (DU). | | Dataset2 |
|---|
Aug 23: LAK add F & G to TX and add G data to GT. | Row | STUDYID | GNTXAID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|
| 1 | 8325064 |
Ames | | TX | SetA | A-TA98-C0 | 1 | SPECIES | Species | SALMONELLA TYPHIMURIUM | | 2 | 8325064 |
Ames | STRAIN | Strain/Substrain | | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 |
TA98AmesMETACT | Metabolic Activation | | IVTDTRG | In vitro Treatment Duration Target | 72 |
NOT APPLICABLEAmes | METACTFL | Y/N presence of metabolic activation | | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 |
NAmesTRTDMIN| IVTDU | In vitro Treatment Duration |
Minimum71.5Ames | TRTDTRG | Treatment Duration Target | | INCBTMP | Incubation Temperature | 37 |
72Ames | TRTDMAX | Treatment Duration Maximum | | INCBTMPU | Incubation Temperature Unit | C |
72.5Ames | TRTDU | Treatment Duration Unit | | ATMRHP | Atmospheric Relative Humidity Percent | 50 |
HOURSAmes | INCBTMP | Incubation Temperature | | ATMCO2P | Atmospheric CO2 Percent | 5 |
37Ames | INCBTMPU | Incubation Temperature Unit | | SPTOBID | Applicant-defined tobacco identifier | CIG01a |
CAmesHUMID | Atmospheric Relative Humidity Percent | 50Ames | ATMCO2 | Atmospheric CO2 Percent | | SAMTYP | Sample Type | Total Particulate Matter in PBS |
5Ames | SPTOBIDSponsor tobacco CIG01aAmesEXPTYP | | Smoking Regimen | NON-INTENSE REGIMEN |
SubmergedAmes | SAMTYP | Sample Type | | STRAIN | Strain/Substrain | TA98 |
Total Particulate Matter in PBSAmes | INTRVN | Name of the intervention article | | MTACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
TA1AmesITVTYPEType of intervention article | | Presence of Metabolic Activation Flag | N |
Vehicle ControlAmes | ITVCONC | Concentration of intervention article | 0| ITVNAM | Intervention Article Name | DMSO | | 19 | 8325064 |
AmesITVCONCU | Concentration Unit | | ITVTYPE | Intervention Article Type | VEHICLE |
ug/plateAmesTRTV | Treatment Vehicle | | ITVCONC | Intervention Article Concentration | 100 |
DMSOAmes | SPDEVID | Sponsor defined device identifier | | ITVCONCU | Intervention Article Concentration Unit | % |
PUFFMASTER3KAmes | DUREFIDSmoke Regimen | NON-INTENSE REGIMENAmes | | TX | SetF | F-TA98-C500 | 23 | SPECIES | Species | SALMONELLA TYPHIMURIUM | | 24 | 8325064 |
AmesSTRAIN | Strain/Substrain | | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 |
TA98AmesMETACT | Metabolic Activation | | IVTDTRG | In vitro Treatment Duration Target | 72 |
NOT APPLICABLEAmes | METACTFL | Y/N presence of metabolic activation | | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 |
NAmes | TRTDMIN| IVTDU | In vitro Treatment Duration |
Minimum71.5Ames | TRTDTRG | Treatment Duration Target | | INCBTMP | Incubation Temperature | 37 |
72AmesTRTDMAX | Treatment Duration Maximum | | INCBTMPU | Incubation Temperature Unit | C |
72.5Ames | TRTDU | Treatment Duration Unit | | ATMRHP | Atmospheric Relative Humidity Percent | 50 |
HOURSAmesINCBTMP | Incubation Temperature | | ATMCO2P | Atmospheric CO2 Percent | 5 |
37Ames | INCBTMPU | Incubation Temperature Unit | | SPTOBID | Applicant-defined tobacco identifier | CIG01a |
CAmesHUMID | Atmospheric Relative Humidity Percent | 50Ames | ATMCO2 | Atmospheric CO2 Percent | | SAMTYP | Sample Type | Total Particulate Matter in PBS |
5AmesSPTOBIDSponsor tobacco CIG01aAmes | EXPTYP | | Smoking Regimen | NON-INTENSE REGIMEN |
SubmergedAmesSAMTYP | Sample Type | | STRAIN | Strain/Substrain | TA98 |
Total Particulate Matter in PBSAmesINTRVNName of the intervention article | | Metabolic Activating Agent Name | NOT APPLICABLE |
TA1Ames | ITVTYPEType of intervention article | | Presence of Metabolic Activation Flag | N |
Vehicle ControlAmes | ITVCONC | Concentration of intervention article | | ITVNAM | Intervention Article Name | TA1 |
500Ames | ITVCONCU | Concentration Unit | | ITVTYPE | Intervention Article Type | PRODUCT |
ug/plateAmesTRTV | Treatment Vehicle | | ITVCONC | Intervention Article Concentration | 500 |
DMSOAmesSPDEVID | Sponsor defined device identifier | | ITVCONCU | Intervention Article Concentration Unit | ug/plate |
PUFFMASTER3KAmes | DUREFIDSmoke Regimen | NON-INTENSE REGIMENAmes | | TX | SetG | G-TA98-C1600 | 45 | SPECIES | Species | SALMONELLA TYPHIMURIUM | | 46 | 8325064 |
Ames | STRAIN | Strain/Substrain | | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 |
TA98AmesMETACT | Metabolic Activation | | IVTDTRG | In vitro Treatment Duration Target | 72 |
NOT APPLICABLEAmes | METACTFL | Y/N presence of metabolic activation | | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 |
NAmes | TRTDMIN| IVTDU | In vitro Treatment Duration |
Minimum71.5AmesTRTDTRG | Treatment Duration Target | | INCBTMP | Incubation Temperature | 37 |
72AmesTRTDMAX | Treatment Duration Maximum | | INCBTMPU | Incubation Temperature Unit | C |
72.5Ames | TRTDU | Treatment Duration Unit | | ATMRHP | Atmospheric Relative Humidity Percent | 50 |
HOURSAmesINCBTMP | Incubation Temperature | | ATMCO2P | Atmospheric CO2 Percent | 5 |
37AmesINCBTMPU | Incubation Temperature Unit | | SPTOBID | Applicant-defined tobacco identifier | CIG01a |
CAmesHUMID | Atmospheric Relative Humidity Percent | 50Ames | ATMCO2 | Atmospheric CO2 Percent | | SAMTYP | Sample Type | Total Particulate Matter in PBS |
5Ames | SPTOBID | | 57 | APDEVID | Applicant-defined device |
Sponsor defined tobacco CIG01aAmes | EXPTYP | | | 58 | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN |
SubmergedAmesSAMTYP | Sample Type | | STRAIN | Strain/Substrain | TA98 |
Total Particulate Matter in PBSAmesINTRVN | Name of the intervention article | | 60 | MTACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
TA1Ames | ITVTYPEType of intervention article | | Presence of Metabolic Activation Flag | N |
Vehicle ControlAmes | ITVCONC | Concentration of intervention article | | 62 | ITVNAM | Intervention Article Name | TA1 |
1600Ames | ITVCONCU | Concentration Unit | | 63 | ITVTYPE | Intervention Article Type | Product |
ug/plateAmesTRTV | Treatment Vehicle | | ITVCONC | Intervention Article Concentration | 1600 |
DMSOAmes | SPDEVID | Sponsor defined device identifier | | ITVCONCU | Intervention Article Concentration Unit | ug/plate |
PUFFMASTER3KAmes | DUREFID | Smoke Regimen | | 66 | TRTV | Treatment Vehicle | DMSO |
NON-INTENSE REGIMENAmes | TX | SetR | R-TA100-C2 | 67 | SPECIES | Species |
Salmonella typhimurium| SALMONELLA TYPHIMURIUM | | 68 | 8325064 |
Ames | STRAIN | Strain/Substrain | TA100 | | 68 | IVTDMIN | In vitro Treatment Duration Minimum | 71.5 | | 69 | 8325064 |
AmesMETACT | Metabolic Activation | | IVTDTRG | In vitro Treatment Duration Target | 72 |
NOT APPLICABLEAmesMETACTFL | Y/N presence of metabolic activation | | 70 | IVTDMAX | In vitro Treatment Duration Maximum | 72.5 |
NAmes | TRTDMIN| IVTDU | In vitro Treatment Duration |
Minimum71.5AmesTRTDTRG | Treatment Duration Target | | 72 | INCBTMP | Incubation Temperature | 37 |
72Ames | TRTDMAX | Treatment Duration Maximum | | 73 | INCBTMPU | Incubation Temperature Unit | C |
72.5AmesTRTDU | Treatment Duration Unit | | 74 | ATMRHP | Atmospheric Relative Humidity Percent | 50 |
HOURSAmesINCBTMP | Incubation Temperature | | 75 | ATMCO2P | Atmospheric CO2 Percent | 5 |
37AmesINCBTMPU | Incubation Temperature Unit | | SPTOBID | Applicant-defined tobacco identifier | CIG01a |
CAmesHUMID | Atmospheric Relative Humidity Percent | 50AmesATMCO2 | Atmospheric CO2 Percent | | SAMTYP | Sample Type | Total Particulate Matter in PBS |
5Ames | SPTOBIDSponsor tobacco CIG01aAmesEXPTYP | | | 80 | SMKRGM | Smoking Regimen | NON-INTENSE REGIMEN |
SubmergedAmesSAMTYP | Sample Type | | 81 | STRAIN | Strain/Substrain | TA100 |
Total Particulate Matter in PBSAmes | INTRVN | name of the intervention article | | MTACTIND | Metabolic Activating Agent Name | NOT APPLICABLE |
TA1AmesITVTYPEtype of intervention article | | Presence of Metabolic Activation Flag | N |
Positive ControlAmes | ITVCONC | Concentration of intervention article | | ITVNAM | Intervention Article Name | NaN3 |
2Ames | ITVCONCU | Concentration Unit | | ITVTYPE | Intervention Article Type | Positive Control |
ug/plateAmes | TRTV | Treatment Vehicle | | ITVCONC | Intervention Article Concentration | 2 |
DMSOAmesSPDEVID | Sponsor defined device identifier | | 87 | ITVCONCU | Intervention Article Concentration Unit | ug/plate |
PUFFMASTER3KAmesDUREFIDSmoke Regimen | NON-INTENSE REGIMEN |
|
REFID values are defined by the applicant to uniquely identify the observational unit within an experimental unit. In the simplified picture diagram of an assay procedure (below), the test tube that contains the possible mutagen is an example of an entity that would have a REFID indicating information at the level of trial set. The petri plate created from that test tube and used to count revertant numbers is an example of an entity that would be at an observational level.
This picture does not depict the more complicated procedures, trial sets, or observational units for our the example study, 8325064. For For study 8325064, the applicant chose to define the REFIDs at the trial set level with a single character (e.g., A, F, G, R) and chose to define REFIDs at the observational level based on the location of each tube in one 1 of multiple exposure/incubation plates (e.g, 6_1 for plate 6, position 1).

| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Row 1: | Shows the value of REFID=A |
, . This REFID refers to the trial set with a SETCD of |
'' trial set dataset, tx.xpt. TX dataset. LEVEL=1 and LVLDESC= |
'"EXPERIMENTAL UNIT/TRIAL SET". TRIAL SET |
' indicates this identifier relates to the entire trial set. EXPERIMENTAL UNIT indicates this identifier received the intervention article, DMSO. | | Rows 2-6: | Show the values of |
five 0 0| 1_5) that are within the parent experimental unit, REFID=A. | | Row 7: | Shows the value of REFID=F |
, | . This REFID refers to the trial set with a SETCD of |
'' trial set , txxpt. and LVLDESC='| and LVLDESC="EXPERIMENTAL UNIT/TRIAL SET". TRIAL SET |
' | indicates this identifier relates to the entire trial set. EXPERIMENTAL UNIT indicates this identifier received the intervention article, TA1. | | Rows 8-10: | Show the values of |
three | 3 observational units (6_1 through 6_3) that are within the parent experimental unit, REFID=F. | | Row 11: | Shows the value of REFID=G |
, | . This REFID refers to the trial set with a SETCD of |
'' trial set , txxpt. '| "EXPERIMENTAL UNIT/TRIAL SET". TRIAL SET |
' | indicates this identifier relates to the entire trial set. EXPERIMENTAL UNIT indicates this identifier received the intervention article, TA1. | | Rows 12-14: | Show the values of |
three | 3 observational units (7_1 through 7_3) that are within the parent experimental unit, REFID= |
F| G. | | Row 15: | Shows the value of REFID=R. This REFID refers to the trial set with a SETCD of |
'' trial set dataset, tx.xpt. 1 '| "EXPERIMENTAL UNIT/TRIAL SET". TRIAL SET |
' | indicates this identifier relates to the entire trial set. EXPERIMENTAL UNIT indicates this identifier received the intervention article, NaN3. | | Rows 16-18: | |
three 3 observational units (18_1 through 18_3) that are within the parent experimental unit, REFID= |
F |
| Dataset2 |
|---|
| |
|---|
| Row | STUDYID | SETCD | REFID | PARENT | LEVEL | LVLDESC |
|---|
1 | 8325064 | SetA | A |
| 1 | EXPERIMENTAL UNIT/TRIAL SET |
|---|
2 | 8325064 | SetA | 1_1 | A | 2 | OBSERVATIONAL UNIT |
|---|
3 | 8325064 | SetA | 1_2 | A | 2 | OBSERVATIONAL UNIT |
|---|
4 | 8325064 | SetA | 1_3 | A | 2 | OBSERVATIONAL UNIT |
|---|
5 | 8325064 | SetA | 1_4 |
|---|
Row | STUDYID | GNTXAID | SETCD | REFID | PARENT | LEVEL | LVLDESC |
|---|
1 | 8325064 | Ames | SetA | A | 1 | TRIAL SET | 2 | 8325064 | Ames | SetA | 0_1 | A | 2 | OBSERVATIONAL UNIT | 36 | 8325064 |
|---|
Ames | SetA | 01_ | 25 | A | 2 | OBSERVATIONAL UNIT | 47 | 8325064 |
|---|
Ames | SetA | 0_3 | ASetF | F |
| 1 | EXPERIMENTAL UNIT/TRIAL SET | 8 | 8325064 | SetF | 6_1 | F | 2 | OBSERVATIONAL UNIT |
|---|
59 | 8325064 |
|---|
Ames| SetF | SetA | 0| 6_ | 4| 2 | A| F | 2 | OBSERVATIONAL UNIT | 610 | 8325064 |
|---|
AmesSetF | SetA | 0| 6_ | 5| 3 | A| F | 2 | OBSERVATIONAL UNIT | 711 | 8325064 |
|---|
AmesSetG | SetFG | F |
| 1 | EXPERIMENTAL UNIT/TRIAL SET | 812 | 8325064 |
|---|
Ames6| SetG | SetF | | 7_1 | F| G | 2 | OBSERVATIONAL UNIT | 913 | 8325064 |
|---|
AmesSetG | SetF | 6| 7_2 | F| G | 2 | OBSERVATIONAL UNIT | 10| 14 | 8325064 | Ames6| SetG | SetF | | 7_3 | F| G | 2 | OBSERVATIONAL UNIT | 11| 15 | 8325064 | AmesSetR | SetGR | G |
| 1 | EXPERIMENTAL UNIT/TRIAL SET | 12| 16 | 8325064 | Ames7| SetR | SetG | | 18_1 | G| R | 2 | OBSERVATIONAL UNIT | 13| 17 | 8325064 | Ames| SetR | SetG | 7| 18_2 | G| R | 2 | OBSERVATIONAL UNIT | 14| 18 | 8325064 | Ames7| SetR | SetG | | 18_3 | G| R | 2 | OBSERVATIONAL UNIT | 15
|
|
| Dataset wrap |
|---|
8325064 | Ames | SetG | R | 1 | TRIAL SET |
| 16 | 8325064 | Ames | SetR | 18_1 | R | 2 | OBSERVATIONAL UNIT |
| 17 | 8325064 | Ames | SetR | 18_2 | R | 2 | OBSERVATIONAL UNIT |
| 18 | 8325064 | Ames | SetR | 18_3 | R | 2 | OBSERVATIONAL UNIT |
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Rows 1-5: | Show the number of revertant colonies per plate collected for each of 5 observational units, GTREFID=1_1 through 1_5 (see description in the RELREF dataset). | | Rows 6, 7: | Show summary values collected (mean, standard deviation) for GTREFID=A that apply to the entire trial set, SetA, as indicated by LEVEL=1 and LVLDESC=TRIAL SET for this REFID as shown in the RELREF dataset. | | Rows 8-13: | Revertent colonies were counted for each of 3 plates/observational units (GTREFID=6_1 through 6_3) and each value is associated with a record to show a postfix code of "V" (Very thin background bacterial lawn). | | Rows 14-16 |
| | Rowcaps |
|---|
| Rows 1-5: | Show the number of revertant colonies per plate collected for each of five observational units GTREFID=0_1 through 0_5 (see description in the relref dataset). | | Rows 6, 7: | Show summary values collected (MEAN, STANDARD DEVIATIONmean, standard deviation, fold increase) for GTREFID=A F that apply to the entire trial set, SetASetF, as indicated by LEVEL=1 and LVLDESC=TRIAL SET for this REFID as shown in the relref RELREF dataset. | | Rows 817-1319: | Revertent colonies were counted for each of three Show 3 plates/observational units (GTREFID= where no revertant colonies were counted due to too much cytotoxicity and a postfix code of " | | Rows 14-16: | Show summary values collected (MEAN, STANDARD DEVIATION, and FOLD INCREASE) for GTREFID=F that apply to the entire trial set, SetF, as indicated by LEVEL=1 and LVLDESC=TRIAL SET for this REFID in the relref dataset. | T". | Jira |
|---|
| showSummary | false |
|---|
| server | Issue Tracker (JIRA) |
|---|
| columnIds | issuekey,summary,issuetype,created,updated,duedate,assignee,reporter,priority,status,resolution |
|---|
| columns | key,summary,type,created,updated,due,assignee,reporter,priority,status,resolution |
|---|
| serverId | 85506ce4-3cb3-3d91-85ee-f633aaaf4a45 |
|---|
| key | TOBA-668 |
|---|
|
| Rows 17-19: | Show three plates/observational units (GTREFID=5_1 through 5_3) where no revertant colonies were counted due to too much cytotoxicity and a postfix code of "T". | | Rows 20-22: | Show the number of revertant colonies per plate collected for each of three 3 observational units (GTREFID=18_1 through 18_3). | | Rows 23-25: | Show summary values collected (MEANmean, STANDARD DEVIATION, and FOLD INCREASEstandard deviation, fold increase) for GTREFID=R that apply to the entire trial set, SetR, as indicated by LEVEL=1 and LVLDESC=TRIAL SET for this REFID in the relref RELREF dataset. |
|
| Dataset2 |
|---|
GNTXAID | DOMAIN | GTSEQ | GTREFID | GTTESTCD | GTTEST | GTORRES | GTORRESU | GTCOLSRT | GTSTRESC | GTSTRESN | GTSTRESU | GTSTAT | GTREASND | GTMETHOD | GTDTC |
|---|
| 1 | 8325064 |
Ames | 0| 1_1 | RPP | Revertant Colony Numbers Per Plate | 26 |
NOT APPLICABLE | NOT APPLICABLE | |
|
| INSTRUMENT COUNTED | 2015-08-03 | | 2 | 8325064 |
Ames | 0| 1_2 | RPP | Revertant Colony Numbers Per Plate | 35 |
NOT APPLICABLE | NOT APPLICABLE |
|
|
| INSTRUMENT COUNTED | 2015-08-03 | | 3 | 8325064 |
Ames | 0| 1_3 | RPP | Revertant Colony Numbers Per Plate | 39 |
NOT APPLICABLE | NOT APPLICABLE |
|
|
| INSTRUMENT COUNTED | 2015-08-03 | | 4 | 8325064 |
Ames | 0| 1_4 | RPP | Revertant Colony Numbers Per Plate | 35 |
NOT APPLICABLE | NOT APPLICABLE |
|
|
| INSTRUMENT COUNTED | 2015-08-03 | | 5 | 8325064 |
Ames | 0| 1_5 | RPP | Revertant Colony Numbers Per Plate | 30 |
NOT APPLICABLE | NOT APPLICABLE |
|
|
| INSTRUMENT COUNTED | 2015-08-03 | | 6 | 8325064 |
Ames | | GT | 6 | A | RPP | Revertant Colony Numbers Per Plate | 33.0 |
NOT APPLICABLE | NOT APPLICABLEAmes | GT | 7 | A | RPP | Revertant Colony Numbers Per Plate | 5.0 |
NOT APPLICABLENOT APPLICABLE | Ames1| 8 | 6_1 | RPP | Revertant Colony Numbers Per Plate | 8 |
NOT APPLICABLENOT APPLICABLE |
|
|
| MANUALLY COUNTED | 2015-08-03 | | 9 | 8325064 |
Ames2| 9 | 6_1 | CYTOTOX | Cytotoxicity | Very thin background bacterial lawn |
|
| V |
|
|
|
|
| 2015-08-03 | | 10 | 8325064 |
Ames | 3| 10 | 6_2 | RPP | Revertant Colony Numbers Per Plate | 10 |
NOT APPLICABLE | NOT APPLICABLE |
|
|
| MANUALLY COUNTED | 2015-08-03 | | 11 | 8325064 |
Ames | 4| 11 | 6_2 | CYTOTOX | Cytotoxicity | Very thin background bacterial lawn |
|
| V |
|
|
|
|
| 2015-08-03 | | 12 | 8325064 |
Ames | 5| 12 | 6_3 | RPP | Revertant Colony Numbers Per Plate | 12 |
NOT APPLICABLE | NOT APPLICABLE |
|
|
| MANUALLY COUNTED | 2015-08-03 | | 13 | 8325064 |
Ames | 6| 13 | 6_3 | CYTOTOX | Cytotoxicity | Very thin background bacterial lawn |
|
| V |
|
|
|
|
| 2015-08-03 | | 14 | 8325064 |
Ames7| 14 | F | RPPMEAN | Mean Rev Colony Num Per Plate | 10.0 |
NOT APPLICABLENOT APPLICABLE | Ames | 8| 15 | F | RPPSTDDV | Std Dev Rev Colony Num Per Plate | 2.0 |
NOT APPLICABLENOT APPLICABLE | Ames | 9| 16 | F | RPPFLDIC | Fold Increase Rev Colony Num Per Plate | 0.3 |
NOT APPLICABLE | NOT APPLICABLEAmes | 15| 7_1 | CYTOTOX | Cytotoxicity | Toxic No Revertant Colonies |
|
| T |
|
| NOT DONE | TOO MUCH CYTOTOXICITY |
| 2015-08-03 | | 18 | 8325064 |
Ames | 25| 7_2 | CYTOTOX | Cytotoxicity | Toxic No Revertant Colonies |
|
| T |
|
| NOT DONE | TOO MUCH CYTOTOXICITY |
| 2015-08-03 | | 19 | 8325064 |
Ames35| 7_3 | CYTOTOX | Cytotoxicity | Toxic No Revertant Colonies |
|
| T |
|
| NOT DONE | TOO MUCH CYTOTOXICITY |
| 2015-08-03 | | 20 | 8325064 |
Ames1| 20 | 18_1 | RPP | Revertant Colony Numbers Per Plate | 812 |
NOT APPLICABLENOT APPLICABLE |
|
|
| INSTRUMENT COUNTED | 2015-08-03 | | 21 | 8325064 |
Ames2| 21 | 18_2 | RPP | Revertant Colony Numbers Per Plate | 912 |
NOT APPLICABLENOT APPLICABLE |
|
|
| INSTRUMENT COUNTED | 2015-08-03 | | 22 | 8325064 |
Ames | 3| 22 | 18_3 | RPP | Revertant Colony Numbers Per Plate | 839 |
NOT APPLICABLENOT APPLICABLE |
|
|
| INSTRUMENT COUNTED | 2015-08-03 | | 23 | 8325064 |
Ames4| 23 | R | RPPMEAN | Mean Rev Colony Num Per Plate | 854.3 |
NOT APPLICABLENOT APPLICABLE | Ames | 5| 24 | R | RPPSTDDV | Std Dev Rev Colony Num Per Plate | 51.7 |
NOT APPLICABLE |
| STANDARD DEVIATION | 51.7 | 51.7 |
NOT APPLICABLE | Ames | 6| 25 | R | RPPFLDIC | Fold Increase Rev Colony Num Per Plate | 6.4 |
NOT APPLICABLE | NOT APPLICABLE |
|


