This is an example showing the raw data report table, trial design, and results from the in vitro bacterial reverse mutation test of an example study, Study #8325064. This bacterial reverse mutation test uses four different amino acid-requiring strains of Salmonella typhimurium (S. typhimurium) and one strain of Escherichia coli (E. coli) to detect point mutations, which involve substitution, addition or deletion of one or a few DNA base pairs.
...
In this example, the Trial Summary dataset, ts.xpt, includes many informational fields that may provide context at the study level, for Study 8325064. Also, TSGRPID has been used to link records (name, location, country) related to the test facility (TSGRPID = 1) and records related to the Test Site (TSGRPID = 2). The Study Director is associated with the Test Facility and the Principal Investigator is associated with the Test Site. Finally, the Primary Treatment CAS Registry Number is not known and this is recorded as an empty TSVAL and UNKNOWN in the corresponding TSVALNF.
| Expand |
|---|
| title | Raw Data Report Table for Example Study 8325064 |
|---|
|
This table shows the assay results for Study 8325064, Test Article TA1, and three strains of Salmonella (TA98, TA100, and TA1535) at varying concentrations. For ease of reference, each row has been labeled in the right hand margin with the sponsor defined trial set label (e.g., SetA). For brevity, the remaining tables (e.g., additional strains, samples prepared without metabolic activation, etc.) are not included. 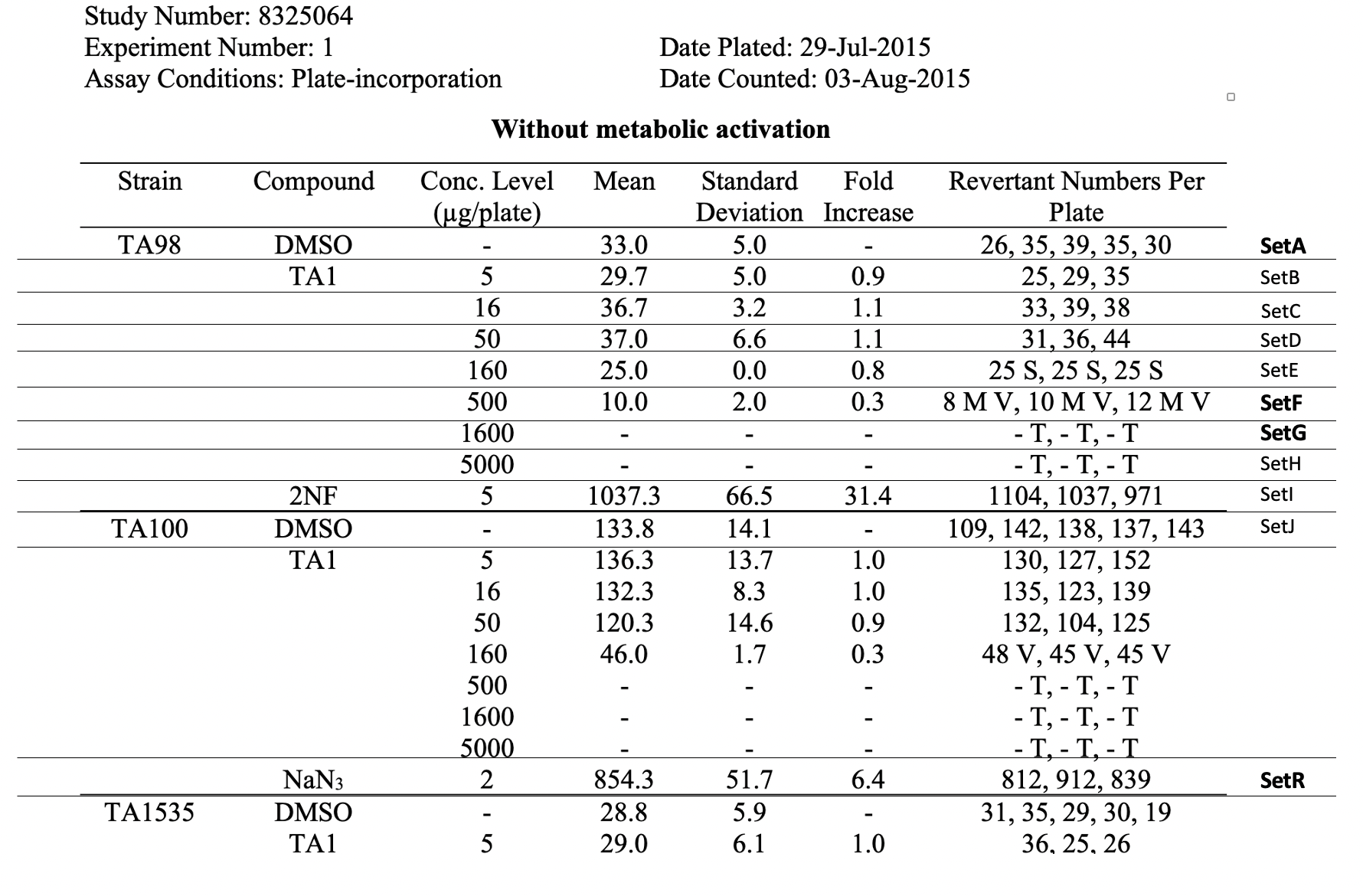
|
...
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Rows 1-22: | Show trial set parameters and trial set values that comprise the test conditions for the set SetA. SetA is the data for the vehicle control (i.e., Strain of TA98, vehicle control with concentration value of 0). SetA is associated with the first row, labeled "SetA", in the report table Raw Data for Study example study 8325064. | | Rows 23-44: | Show trial set parameters and trial set values that comprise the test conditions for the set SetR. SetR is the data for the vehicle control (i.e., Strain of TA100, positive control with a concentration value of 2). SetR is associated with the eighteenth row, labeled "SetR", in the report table Raw Data for Study example study 8325064. |
|
| Dataset2 |
|---|
| Row | STUDYID | ASSAYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|
| 1 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 1 | SPECIES | Species | Salmonella typhimurium | | 2 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 2 | STRAIN | Strain/Substrain | TA98 | | 3 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 3 | METACT | Metabolic Activation |
| | 4 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 4 | METACTFL | Y/N presence of metabolic activation | N | | 5 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 5 | TRTDMIN | Treatment Duration Minimum | 71.5 | | 6 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 6 | TRTDTRG | Treatment Duration Target | 72 | | 7 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 7 | TRTDMAX | Treatment Duration Maximum | 72.5 | | 8 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 8 | TRTDU | Treatment Duration Unit | HOURS | | 9 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 9 | INCBTMP | Incubation Temperature | 37 | | 10 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 10 | INCBTMPU | Incubation Temperature Unit | C | | 11 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 11 | HUMID | Atmospheric Relative Humidity Percent | 50 | | 12 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 12 | ATMCO2 | Atmospheric CO2 Percent | 5 | | 13 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 13 | SPTOBID | Sponsor defined tobacco identifier | CIG01a | | 14 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 14 | EXPTYP | Exposure Type (See TIG NC workstream minutes 30-Jan here: Nonclinical) | Submerged | | 15 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 15 | SAMTYP | Sample Type | Total Particulate Matter in PBS | | 16 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 16 | INTRVN | name of the intervention article | TA1 | | 17 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 17 | ITVTYPE | type of intervention article | Vehicle Control | | 18 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 18 | ITVCONC | Concentration of intervention article | 0 | | 19 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 19 | ITVCONCU | Concentration Unit | ug/plate | | 20 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 20 | TRTV | Treatment Vehicle | DMSO | | 21 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 21 | SPDEVID | Sponsor defined device identifier | PUFFMASTER3K | | 22 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 22 | DUREFID | Smoke Regimen | Medium Intensity Regimen | | 23 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 1 | SPECIES | Species | Salmonella typhimurium | | 24 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 2 | STRAIN | Strain/Substrain | TA100 | | 25 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 3 | METACT | Metabolic Activation |
| | 26 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 4 | METACTFL | Y/N presence of metabolic activation | N | | 27 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 5 | TRTDMIN | Treatment Duration Minimum | 71.5 | | 28 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 6 | TRTDTRG | Treatment Duration Target | 72 | | 29 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 7 | TRTDMAX | Treatment Duration Maximum | 72.5 | | 30 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 8 | TRTDU | Treatment Duration Unit | HOURS | | 31 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 9 | INCBTMP | Incubation Temperature | 37 | | 32 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 10 | INCBTMPU | Incubation Temperature Unit | C | | 33 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 11 | HUMID | Atmospheric Relative Humidity Percent | 50 | | 34 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 12 | ATMCO2 | Atmospheric CO2 Percent | 5 | | 35 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 13 | SPTOBID | Sponsor defined tobacco identifier | CIG01a | | 36 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 14 | EXPTYP | | Submerged | | 37 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 15 | SAMTYP | Sample Type | Total Particulate Matter in PBS | | 38 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 16 | INTRVN | name of the intervention article | TA1 | | 39 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 17 | ITVTYPE | type of intervention article | Positive Control | | 40 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 18 | ITVCONC | Concentration of intervention article | 2 | | 41 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 19 | ITVCONCU | Concentration Unit | ug/plate | | 42 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 20 | TRTV | Treatment Vehicle | DMSO | | 43 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 21 | SPDEVID | Sponsor defined device identifier | PUFFMASTER3K | | 44 | 8325064 | Ames | TX | SetR | R-TA100-C2 | 22 | DUREFID | Smoke Regimen | Medium Intensity Regimen |
|
|
...

