...
| Expand |
|---|
| title | Raw Data Table for Study 8325064 |
|---|
|
|
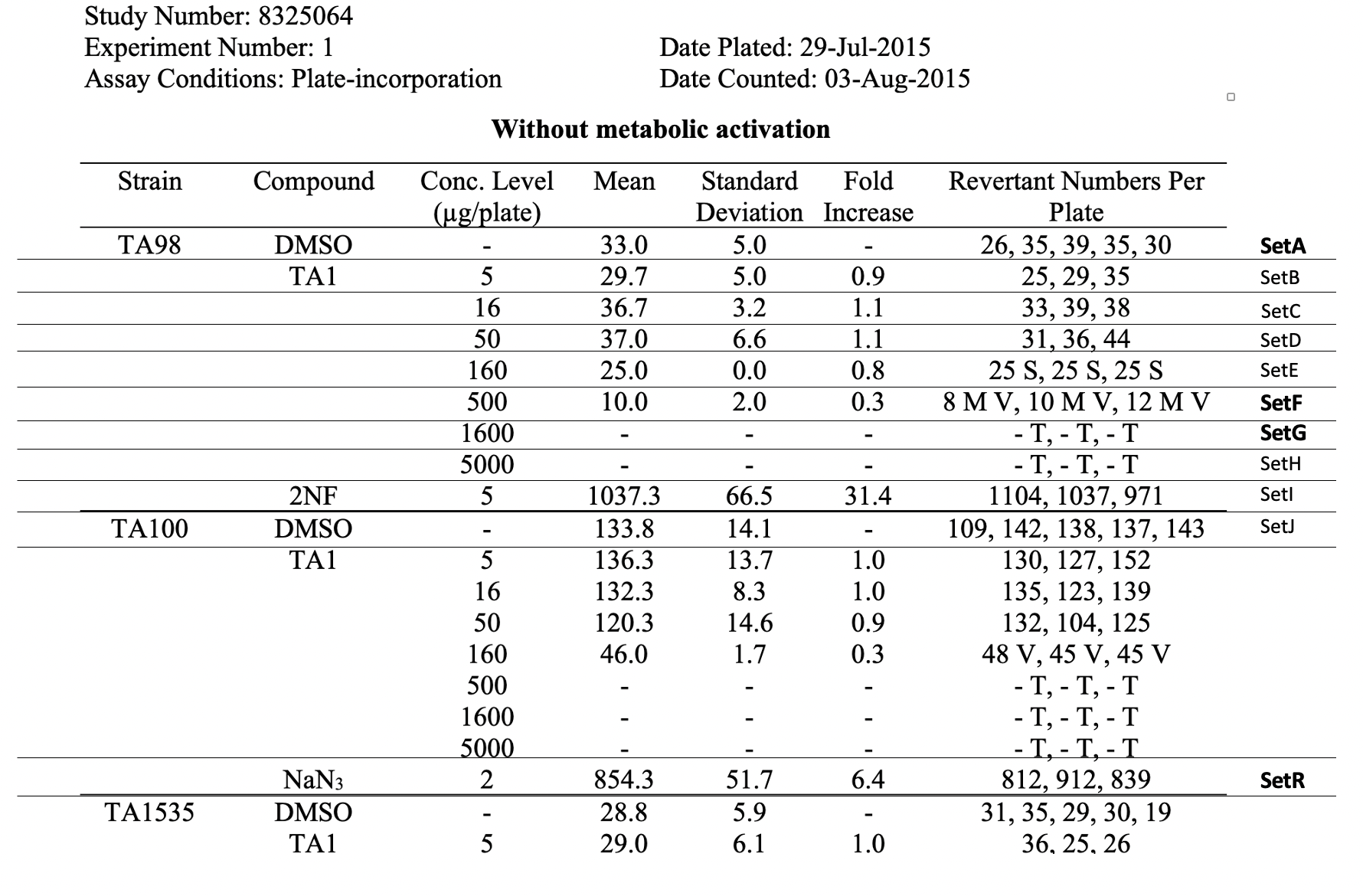
This example trial set dataset shows information about the test conditions for SetA and SetR in this example study. For brevity, this does not show information for SetF, SetG, or any other sets. A fully formed tx.xpt for this example study would include information about the test conditions for all sets.
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Rows 1-22: | Show trial set parameters and trial set values that comprise the test conditions for the set SetA. SetA is the data for the vehicle control (i.e., a concentration level of 0) as shown in the first row, labeled "SetA, Strain of TA98, vehicle control with concentration value of 0). SetA is associated with the first row, labeled "SetA", in the table Raw Data for Study 8325064. | | Rows 23-44: | Show trial set parameters and trial set values that comprise the test conditions for the set SetR. SetR is the data for the vehicle control (i.e., Strain of TA100, positive control with a concentration value of 2). SetR is associated with the eighteenth row, labeled "SetR", in the table Raw Data for Study 8325064. | Rows 23-4: | Rows 6-7: | Row 13: | Rows 1, 6: |
|
| Dataset2 |
|---|
| Row | STUDYID | ASSAYID | DOMAIN | SETCD | SET | TXSEQ | TXPARMCD | TXPARM | TXVAL |
|---|
| 1 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 1 | SPECIES | Species | Salmonella typhimurium | | 2 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 2 | STRAIN | Strain/Substrain | TA98 | | 3 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 3 | METACT | Metabolic Activation |
| | 4 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 4 | METACTFL | Y/N presence of metabolic activation | N | | 5 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 5 | TRTDMIN | Treatment Duration Minimum | 71.5 | | 6 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 6 | TRTDTRG | Treatment Duration Target | 72 | | 7 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 7 | TRTDMAX | Treatment Duration Maximum | 72.5 | | 8 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 8 | TRTDU | Treatment Duration Unit | HOURS | | 9 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 9 | INCBTMP | Incubation Temperature | 37 | | 10 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 10 | INCBTMPU | Incubation Temperature Unit | C | | 11 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 11 | HUMID | Atmospheric Relative Humidity Percent | 50 | | 12 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 12 | ATMCO2 | Atmospheric CO2 Percent | 5 | | 13 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 13 | SPTOBID | Sponsor defined tobacco identifier | CIG01a | | 14 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 14 | EXPTYP | Exposure Type (See TIG NC workstream minutes 30-Jan here: Nonclinical) | Submerged | | 15 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 15 | SAMTYP | Sample Type | Total Particulate Matter in PBS | | 16 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 16 | INTRVN | name of the intervention article | TA1 | | 17 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 17 | ITVTYPE | type of intervention article | Vehicle Control | | 18 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 18 | ITVCONC | Concentration of intervention article | 0 | | 19 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 19 | ITVCONCU | Concentration Unit | ug/plate | | 20 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 20 | TRTV | Treatment Vehicle | DMSO | | 21 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 21 | SPDEVID | Sponsor defined device identifier | PUFFMASTER3K | | 22 | 8325064 | Ames | TX | SetA | A-TA98-C0 | 22 | DUREFID | Smoke Regimen | Medium Intensity Regimen | | 23 | 8325064 | Ames | TX | SetFSetR | FR-TA98TA100-C500C2 | 1 | SPECIES | Species | Salmonella typhimurium | | 24 | 8325064 | Ames | TX | SetFSetR | FR-TA98TA100-C500C2 | 2 | STRAIN | Strain/Substrain | TA98TA100 | | 25 | 8325064 | Ames | TX | SetFSetR | FR-TA98TA100-C500C2 | 3 | METACT | Metabolic Activation |
| | 26 | 8325064 | Ames | TX | SetFSetR | FR-TA98TA100-C500C2 | 4 | METACTFL | Y/N presence of metabolic activation | N | | 27 | 8325064 | Ames | TX | SetF SetR | FR-TA98TA100-C500C2 | 5 | TRTDMIN | Treatment Duration Minimum | 71.5 | | 28 | 8325064 | Ames | TX | SetF SetR | FR-TA98TA100-C500C2 | 6 | TRTDTRG | Treatment Duration Target | 72 | | 29 | 8325064 | Ames | TX | SetF SetR | FR-TA98TA100-C500C2 | 7 | TRTDMAX | Treatment Duration Maximum | 72.5 | | 30 | 8325064 | Ames | TX | SetF SetR | FR-TA98TA100-C500C2 | 8 | TRTDU | Treatment Duration Unit | HOURS | | 31 | 8325064 | Ames | TX | SetF SetR | FR-TA98TA100-C500C2 | 9 | INCBTMP | Incubation Temperature | 37 | | 32 | 8325064 | Ames | TX | SetF SetR | FR-TA98TA100-C500C2 | 10 | INCBTMPU | Incubation Temperature Unit | C | | 33 | 8325064 | Ames | TX | SetF SetR | FR- | TA98TA100- | C500C2 | 11 | HUMID | Atmospheric Relative Humidity Percent | 50 | | 34 | 8325064 | Ames | TX | SetF SetR | FR- | TA98TA100- | C500C2 | 12 | ATMCO2 | Atmospheric CO2 Percent | 5 | | 35 | 8325064 | Ames | TX | SetF SetR | FR- | TA98TA100- | C500C2 | 13 | SPTOBID | Sponsor defined tobacco identifier | CIG01a | | 36 | 8325064 | Ames | TX | SetF SetR | FR- | TA98TA100- | C500C2 | 14 | EXPTYP | Exposure Type (See TIG NC workstream minutes 30-Jan here: Nonclinical) | Submerged | | 37 | 8325064 | Ames | TX | SetF SetR | FR- | TA98TA100- | C500C2 | 15 | SAMTYP | Sample Type | Total Particulate Matter in PBS | | 38 | 8325064 | Ames | TX | SetF SetR | FR- | TA98TA100- | C500C2 | 16 | INTRVN | name of the intervention article | TA1 | | 39 | 8325064 | Ames | TX | SetF SetR | FR- | TA98TA100- | C500C2 | 17 | ITVTYPE | type of intervention article | Vehicle Positive Control | | 40 | 8325064 | Ames | TX | SetF SetR | FR- | TA98TA100- | C500C2 | 18 | ITVCONC | Concentration of intervention article | 02 | | 41 | 8325064 | Ames | TX | SetF SetR | FR- | TA98TA100- | C500C2 | 19 | ITVCONCU | Concentration Unit | ug/plate | | 42 | 8325064 | Ames | TX | SetF SetR | FR- | TA98TA100- | C500C2 | 20 | TRTV | Treatment Vehicle | DMSO | | 43 | 8325064 | Ames | TX | SetF SetR | FR- | TA98TA100- | C500C2 | 21 | SPDEVID | Sponsor defined device identifier | PUFFMASTER3K | | 44 | 8325064 | Ames | TX | SetF SetR | FR- | TA98TA100- | C500C2 | 22 | DUREFID | Smoke Regimen | Medium Intensity Regimen | | 45 |
|
|
|
|
|
|
|
|
| | 46 |
|
|
|
|
|
|
|
|
| | 47 |
|
|
|
|
|
|
|
|
| | 48 |
|
|
|
|
|
|
|
|
|
| 8325064 | Ames | TXSetG (row 7) do G not I!!! |
|
|
| METACT | Metabolic Activation (should there be two parms? Presence, type)? | None |
| 8325064 | Ames | TXSetG |
|
|
| TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) |
|
| 8325064 | Ames | TXSetG |
|
|
| TRTDRTOL | Treatment Duration Tolerance |
|
| 8325064 | Ames | TXSetG |
|
|
| TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) |
|
| 8325064 | Ames | TX | SetG |
|
|
| INTRVN | name of the intervention article (Tobacco ProdA, Bleomycin or Cyclophosphamid A) | 2-Nitrofluorine |
| 8325064 | Ames | TX | SetG |
|
|
| ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Positive Control |
| 8325064 | Ames | TX | SetG |
|
|
| ITVCONC | Concentration of intervention article | 5 |
| 8325064 | Ames | TXSetG |
|
|
| ITVCONCU | Concentration Unit | ug/plate |
| 8325064 | Ames | TX | SetG |
|
|
| REGIME | Smoking Regime | ISO | | ... | SetG |
|
|
|
|
|
|
|
|
|
| 8325064 | Ames | TX SetR (Row 18) |
|
|
| METACT | Metabolic Activation (should there be two parms? Presence, type)? | None |
| 8325064 | Ames | TX | SetR |
|
|
| TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) |
|
| 8325064 | Ames | TX | SetR |
|
|
| TRTDRTOL | Treatment Duration Tolerance |
|
| 8325064 | Ames | TXSetR |
|
|
| TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) |
|
| 8325064 | Ames | TX | SetR |
|
|
| INTRVN | name of the intervention article (Tobacco ProdA, Bleomycin or Cyclophosphamid A) | 4-Nitroquinoline-1-oxide |
| 8325064 | Ames | TXSetR |
|
|
| ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Positive Control |
| 8325064 | Ames | TXSetR |
|
|
| ITVCONC | Concentration of intervention article | 2 |
| 8325064 | Ames | TXSetR |
|
|
| ITVCONCU | Concentration Unit | ug/plate |
| 8325064 | Ames | TX | SetR |
|
| STRAIN | Strain/Substrain | TA98 |
| 8325064 | Ames | TX | SetR |
|
| REGIME | Smoking Regime | ISO |
|
|
...

