Page History
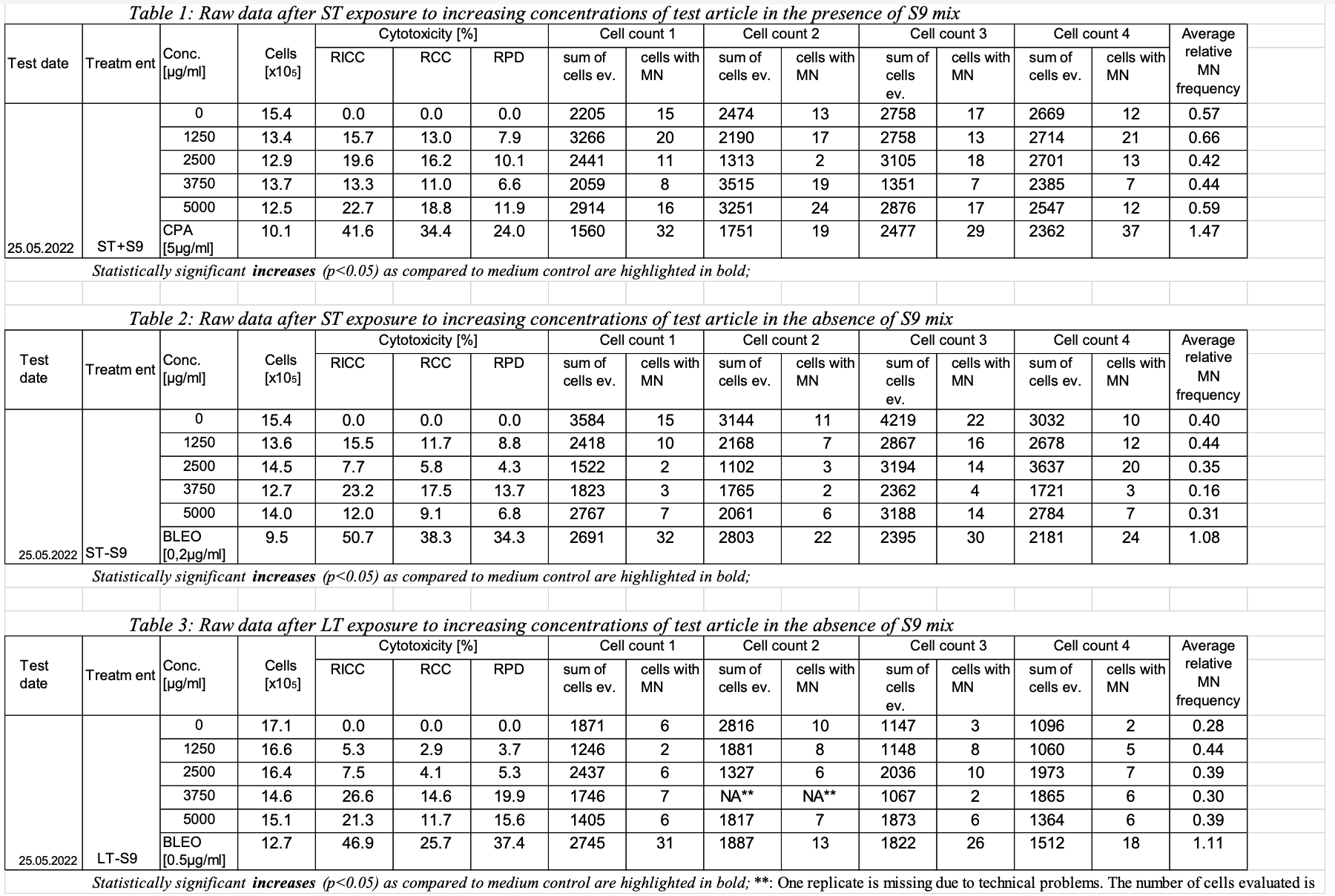
This is an example showing trial design and results data of Study #123 for the determination of the in vitro genotoxicity potential of 10 tobacco products in the in vitro Micronucleus Assay
| Info |
|---|
|
| Expand | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Expand | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Row | STUDYID | ASSAYID | DOMAIN | TSSEQ | TSGRPID | TSPARMCD | TSPARM | TSVAL | TSVALNF | 1 | 123 | MNvit | TS | 1 | GLPTYP | Good Laboratory Practice Type | FDA | 2 | 123 | MNvit | TS | 2 | GLPTYP | Good Laboratory Practice Type | OECD | 3 | 123 | MNvit | TS | 1 | STSTDTC | Study Start Date | 2022-05-25 | 4 | 123 | MNvit | TS | 1 | STITLE | Study Title | Determination of the in vitro genotoxicity potential of 10 tobacco products in the in vitro Micronucleus Assay | 5 | 123 | MNvit | TS | 1 | SNDIGVER | SEND Implementation Guide Version | TOBACCO IMPLEMENTATION GUIDE VERSION 1.0 | 6 | 123 | MNvit | TS | 1 | SNDCTVER | SEND Controlled Terminology Version | SEND Terminology 2021-09-30 | 7 | 123 | MNvit | TS | 1 | SSPONSOR | Sponsor Organization | Example Sponsor Inc. | 8 | 123 | MNvit | TS | 1 | SPREFID | Sponsor's Study Reference ID | NOT APPLICABLE | 9 | 123 | MNvit | TS | 1 | 1 | TSTFNAM | Test Facility Name | Example Tox Lab Name | 10 | 123 | MNvit | TS | 1 | 1 | TSTFLOC | Test Facility Location | 10 Somewhere Street, Montgomery, AL 10000 | 11 | 123 | MNvit | TS | 1 | 1 | TFCNTRY | Test Facility Country | USA | 12 | 123 | MNvit | TS | 1 | 1 | STDIR | Study Director | Dr. R. Smith | 13 | 123 | MNvit | TS | 1 | GLPFL | GLP Flag | Y | 14 | 123 | MNvit | TS | 1 | ASTD | Assay Standard | OECD Test No. 487 | 15 | 123 | MNvit | TS | 1 | ASTDV | Assay Standard Version | 2016-07-29 | 16 | 123 | MNvit | TS | 1 | SSTYP | Study Type | GENOTOXICITY IN VITRO | 17 | 123 | MNvit | TS | 1 | SSSTYP | Study Sub Type | In Vitro Micronucleus | 18 | 123 | MNvit | TS | 1 | SPECIES | Species | Homo Sapiens | 19 | 123 | MNvit | TS | 1 | ?? | Test System? | TK6 Lymphoblastoid Suspension Cells | 20 | 123 | MNvit | TS | 1 | SPDEVID | Sponsor defined device identifier | PUFFMASTER3K | 21 | 123 | MNvit | TS | 1 | DUREFID | Smoke Regimen | Medium Intensity Regimen | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Expand | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Row | STUDYID | ASSAYID | DOMAIN | SETCD | SET (what sponsor calls it) | TXSEQ | TXPARMCD | TXPARM | TXVAL | 123 | MNvit | TX | A1 (table 1, row 1, ST exposure with S9) | METACT | Metabolic Activation (should there be two parms? Presence, type)? | +S9 | METACTFL | Y/N presence of metabolic activation | 123 | MNvit | TX | A1 | TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) | 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
We use DU for smoking regimen. Note that a separate DI dataset will be needed to show identifying parameters of the "PUFFMASTER3K" smoking machine, but is currently not shown for brevity.
| 123 | MNvit | TX | A1 | TRTDRTOL | Treatment Duration Tolerance | 123 | MNvit | TX | A1 | TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | H | 123 | MNvit | TX | A1 | INTRVN | name of the intervention article (Tobacco ProdA, Bleomycin or Cyclophosphamid A) | Tobacco ProdA | 123 | MNvit | TX | A1 | ITVTYPE | type of intervention article choices of values: product; negative control; positive control | Product | 123 | MNvit | TX | A1 | ITVCONC | Concentration of intervention article | 0 | 123 | MNvit | TX | A1 | ITVCONCU | Concentration Unit | ug/ml | 123 | MNvit | TS | A1 | SPDEVID | Sponsor defined device identifier | PUFFMASTER3K | 123 | MNvit | TS | A1 | DUREFID | Smoke Regimen | Medium Intensity Regimen | 123 | MNvit | TX | A2 (table 1, row 2) | METACT | Metabolic Activation (should there be two parms? Presence, type)? | +S9 | 123 | MNvit | TX | A2 | TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) | 3 | 123 | MNvit | TX | A2 | TRTDRTOL | Treatment Duration Tolerance | 123 | MNvit | TX | A2 | TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | H | 123 | MNvit | TX | A2 | INTRVN | name of the intervention article | Tobacco ProdA | 123 | MNvit | TX | A2 | ITVTYPE | type of intervention article | Product | 123 | MNvit | TX | A2 | ITVCONC | Concentration of i a | 1250 | 123 | MNvit | TX | A2 | ITVCONCU | Concentration Unit | ug/ml | 123 | MNvit | TS | A2 | SPDEVID | Sponsor defined device identifier | PUFFMASTER2023 | 123 | MNvit | TS | A2 | DUREFID | Smoke Regimen | High Intensity Regimen | ... | expand|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
du.xpt
|
| Expand | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
A1: A2:
|