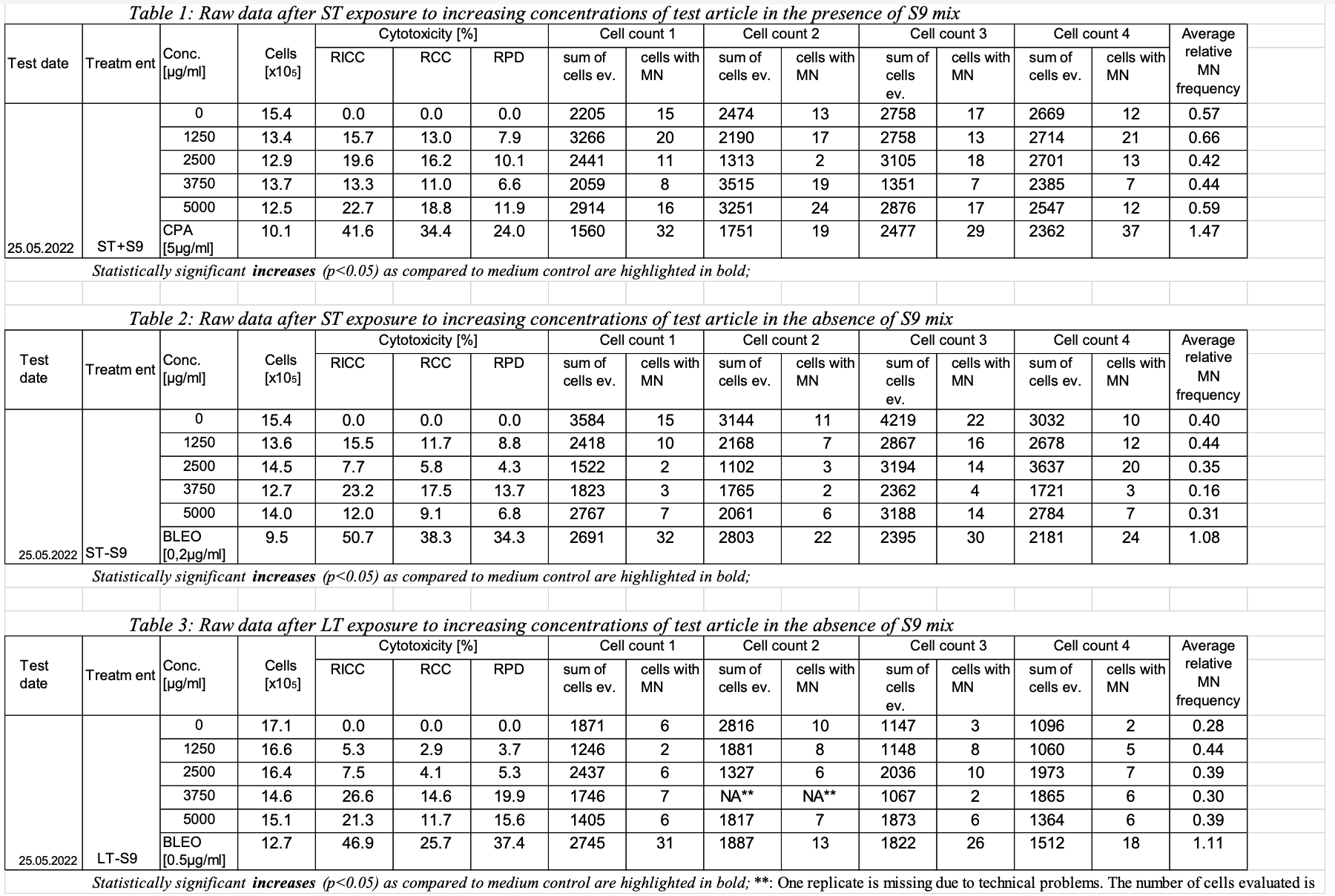
| Row | STUDYID | ASSAYID | DOMAIN | SETCD | | TXSEQ | TXPARMCD | TXPARM | TXVAL | 1 |
|---|
| 123 | NRUMNvit | TX | A1 (table 1 | -1-A(plate-col-row in the 96-well plate)
CSC-10(through SLS-200) | PLATENUM. (e.g., 1 or 2) | 1 | 1 | 2 | 123 | NRU | TX | 1-1-A | CSC-10 | WELLNUM (e.g., 1-A through 10-H) | Number representing the location of the well in the 96-well plate | 1-A | 3 | 123 | NRU | TX | 1-1-A | CSC-10 | INTRVN | name of the intervention article | Cigarette Smoke Condensate | 4 | 123 | NRU | TX | 1-1-A | CSC-10 | ITVTYPE(CT e.g, Product, positive control, negative control) | type of intervention article | PRODUCT | 5 | 123 | NRU | TX | 1-1-A | CSC-10 | ITVCONC | intervention concentration | 10 | 6 | 123 | NRU | TX | 1-1-A | CSC-10 | ITVCONCU | intervention unit | ug/ml | 123 | NRU | 1 | STRAIN | Strain/Substrain | Salmonella enterica enterica | 123 | NRU | 1 | REGIME | Smoking Regime | Traditional combustible | 123 | NRU | 1 | RUN | Assay run number (how is this different from REPNUM) | 1 | 123 | NRU | 1 | PORT | Port ID | 1 | 123 | NRU | 1 | SMPLID | Sample ID | 030001 | 123 | NRU | 1 | SMKFRC | Smoke Fraction | A | 123 | NRU | 1 | REPNUM | Replicate Number | 1 | 11 | 123 | NRU | TX | 1-1-B | CSC-10 | ITVCONC | intervention concentration | 10 | 12 | 123 | NRU | TX | 1-1-B | CSC-10 | ITVCONCU | intervention unit | ug/ml | ... | ... | , row 1, ST exposure with S9) |
|
| METACT | Metabolic Activation (should there be two parms? Presence, type)? | +S9 |
| 123 | MNvit | TX | A1 |
|
| TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) | 3 |
| 123 | MNvit | TX | A1 |
|
| TRTDRTOL | Treatment Duration Tolerance |
|
| 123 | MNvit | TX | A1 |
|
| TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | H |
| 123 | MNvit | TX | A1 |
|
| 123 | NRU | TX | 1-10-H | SLS-10 | PLATENUM | Plate number | 1 | 123 | NRU | TX | 1-10-H | SLS-10 | WELLNUM | Number representing the location of the well in the 96-well plate | 10-H | 123 | NRU | TX | 1-10-H | SLS-10 | INTRVN | name of the intervention article | sodium laurel sulfate(Tobacco ProdA, Bleomycin or Cyclophosphamid A) | Tobacco ProdA |
| 123 | NRUMNvit | TX | 1-10-H | SLS-10A1 |
|
| ITVTYPE | type of intervention article | POSITIVE CONTROL | 123 | NRU | TX | 1-10-H | SLS-10 | ITVCONC | intervention concentration | 200 | 123 | NRU | TX | 1-10-H | SLS-10 | ITVCONCU | intervention unit | ug/ml | ... | ... | choices of values: product; negative control; positive control | Product |
| 123 | MNvit | TX | A1 |
|
| ITVCONC | Concentration of intervention article | 0 |
| 123 | MNvit | TX | A1 |
|
| ITVCONCU | Concentration Unit | 123 | NRU | TX | 2-10-H | SLS-10 | PLATENUM | Plate number | 2 | 123 | NRU | TX | 2-10-H | SLS-10 | WELLNUM | Number representing the location of the well in the 96-well plate | 10-H | 123 | NRU | TX | 2-10-H | SLS-10 | INTRVN | name of the intervention article | sodium laurel sulfate | 123 | NRU | TX | 2-10-H | SLS-10 | ITVTYPE | type of intervention article | POSITIVE CONTROL | 123 | NRU | TX | 2-10-H | SLS-10 | ITVCONC | intervention concentration | 200 | 123 | NRU | TX | 2-10-H | SLS-10 | ITVCONCU | intervention unit | ug/ml |
| 123 | MNvit | TXA1 | A2 (table 1, row 2) |
|
| METACT | Metabolic Activation (should there be two parms? Presence, type)? | +S9 |
| 123 | MNvit | TX | A1A2 |
|
| TRTDRTRG | Treatment Duration target. (how do we show 3-6 hour range? start/end, target and tolerance?, one text field not-analyzable) | 3 |
| 123 | MNvit | TX | 123A2 | MNvit | TX |
|
| TRTDRTOL | Treatment Duration Tolerance |
|
| 123 | MNvit | TX | A2 |
|
| TRTDURU | Treatment Duration Unit (this is for both TRTDURT, TRTDURTOL) | H |
| 123 | MNvit | TX | A2 |
|
| INTRVN | name of the intervention article | Bleomycin or Cyclophosphamid ATobacco ProdA |
| 123 | MNvit | TX | A2 |
|
| ITVTYPE | type of intervention article | choices of values: product; negative control; positive controlProduct |
| 123 | MNvit | TX | A1A2 |
|
| ITVCONC | Concentration of i a | 01250 |
| 123 | MNvit | TX | A1A2 |
|
| ITVCONCU | Concentration Unit | ug/ml |
| ... |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|