| Panel |
|---|
1: Discuss the PCTEST and PCTESTCD Controlled Terminology. They are always measuring nicotine. |
This is an example of a study designed to evaluate plasma nicotine pharmacokinetic (PK) parameters following the use of nicotine pouches. Three different nicotine pouches were used in the study. Each pouch was evaluated in 180 minute test session. AUC and CMAX were to be determined. test session. AUC and CMAX were to be determined.
The study consisted of a screening period, one day of admission, four separate days of on-site product use with 1–3 days in between each product use, and a 7-day safety follow-up period
45, 30, and 15 minutes prior start of product use and 2, 4, 7, 10, 15, 20, 30, 40, 50, 60, 120, and 240 minutes after start of product use.
(Cmax), time to reach Cmax (tmax), and the baseline-corrected area under the plasma concentration–time curves (i) from start of product use (t0) to the last quantifiable nicotine concentration time point (AUC0-last) and (ii) from t0 to 10 minutes after t0 (AUC0-10’). The pharmacokinetic parameters were derived from plasma nicotine concentrations-versus-time data by means of non-compartmental analysis using Phoenix WinNonlin (version 6.2, Pharsight Corp, Sunnyvale, California) and corrected for baseline following the method described by Kraiczi et al.30 whereby the baseline (C0) was defined as the average concentration of the three time points prior to t0 (45, 30, and 15 minutes prior to t0) of each visit from whose slope the elimination rate constant (k) was estimated and then used for the calculation of the baseline corrected values.
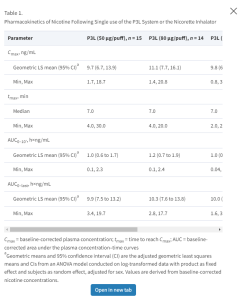 Image Added
Image Added
| Dataset wrap |
|---|
|
| Rowcaps |
|---|
| Rows 1-2: | Show day 1 pre-dose drug and metabolite concentrations in plasma and urine. | | Rows 3-4: | Show day 1 pre-dose drug and metabolite concentrations in urine. Urine specimens may be collected over an interval; both PCDTC and PCENDTC have been populated with the same value to indicate that these specimens were collected at a point in time rather than over an interval. | | Rows 5-6: | Show specimen properties (VOLUME and PH) for the day 1 pre-dose urine specimens. These have a PCCAT value of "SPECIMEN PROPERTY". | | Rows 7-12: | Show day 1 post-dose drug and metabolite concentrations in plasma. | | Rows 13-16: | Show day 11 drug and metabolite concentrations in plasma. | | Rows 17-20: | Show day 11 drug and metabolite concentrations in urine specimens collected over an interval. The elapsed times for urine samples are calculated as the elapsed time (from the reference time point, PCTPTREF) to the end of the specimen collection interval. Elapsed time values that are the same for urine and plasma samples have been assigned the same value for PCTPT. For the urine samples, the value in PCEVLINT describes the planned evaluation (or collection) interval relative to the time point. The actual evaluation interval can be determined by subtracting PCDTC from PCENDTC. | | Rows 21-30: | Show additional drug and metabolite concentrations and specimen properties related to the day 11 dose. | |
| Dataset2 |
|---|
| Row | STUDYID | DOMAIN | USUBJID | PCSEQ | PCGRPID | PCREFID | PCTESTCD | PCTEST | PCCAT | PCSPEC | PCORRES | PCORRESU | PCSTRESC | PCSTRESN | PCSTRESU | PCSTAT | PCLLOQ | PCULOQ | VISITNUM | VISIT | VISITDY | PCDTC | PCENDTC | PCDY | PCTPT | PCTPTNUM | PCTPTREF | PCRFTDTC | PCELTM | PCEVLINT |
|---|
| 1 | ABC-123 | PC | 123-0001 | 1 | Day 1 | A554134-10 | DRGANICO_METPAR | NICOTINE PARENT | ANALYTE | PLASMA | <0.1 | ng/mL | <0.1 |
| ng/mL |
| 0.10 | 20 | 1 | DAY 1 | 1 | 2001-02-01T07:45 |
| 1 | 45MIN PREDOSE | 0 | Day 1 Dose | 2001-02-01T08:00 | -PT15MPT45M |
|
|
|
|
|
|
|
| NICO_PAR | |
| PLASMA | <1.0 |
|
|
|
|
|
|
|
|
|
|
|
|
| 30 MIN PREDOSE |
|
|
| -PT30M |
| | 2 | ABC-123 | PC | 123-0001 | 2 | Day 2 | A554134-10 | DRGA_PAR | Drug A Parent | ANALYTE | PLASMA | <0<1.10 | ng/mL | <0.1 |
| ng/mL |
| 0.10 | 20 | 1 | DAY 1 | 1 | 2001-02-01T07:45 |
| 1 | 15 MIM PREDOSE | 0 | Day 1 Dose | 2001-02-01T08:00 | -PT15M |
| | 3 | ABC-123 | PC | 123-0001 | 3 | Day 3 | A554134-11 | DRGA_MET | Drug A Metabolite | ANALYTE | URINEPLASMA<2 | 1.5 | ng/mL | <2 |
| ng/mL |
| 2.00 | 500 | 1 | DAY 1 | 1 | 2001-02-01T07:45 | 2001-02-01T07:45 | 1 | PREDOSE2M | 0 | Day 1 Dose | 2001-02-01T08:00-PT15M | PT2M |
| | 4 | ABC-123 | PC | 123-0001 | 4 | Day 1 | A554134-11 | DRGA_PAR | Drug A Parent | ANALYTE | URINEPLASMA<2 | 3.0 | ng/mL | <2 |
| ng/mL |
| 2.00 | 500 | 1 | DAY 1 | 1 | 2001-02-01T07:45 | 2001-02-01T07:45 | 1 | PREDOSE4M | 0 | Day 1 Dose | 2001-02-01T08:00-PT15M | PT$M |
| | 5 | ABC-123 | PC | 123-0001 | 5 | Day 1 | A554134-11 | VOLUME | Volume | SPECIMEN PROPERTY | URINEPLASMA3500 | 8.0 | mL | 100 | 100 | mL |
|
|
| 1 | DAY 1 | 1 | 2001-02-01T07:45 | 2001-02-01T07:45 | 1 | PREDOSE8M | 0 | Day 1 Dose | 2001-02-01T08:00-PT15M | PT8M |
| | 6 | ABC-123 | PC | 123-0001 | 6 | Day 1 | A554134-11 | PH | PH | SPECIMEN PROPERTY | URINEPLASMA | 5.0.5 |
| 5.5 | 5.5 |
|
|
|
| 1 | DAY 1 | 1 | 2001-02-01T07:45 | 2001-02-01T07:45 | 1 | PREDOSE60M | 0 | Day 1 Dose | 2001-02-01T08:00-PT15M | PT60 |
| | 7 | ABC-123 | PC | 123-0001 | 7 | Day 1 | A554134-12 | DRGA_MET | Drug A Metabolite | ANALYTE | PLASMA | 53.40 | ng/mL | 5.4 | 5.4 | ng/mL |
| 0.10 | 20 | 1 | DAY 1 | 1 | 2001-02-01T09:30 |
| 1 | 1H30MIN120M | 1.5 | Day 1 Dose | 2001-02-01T08:00 | PT1H30MPT120 |
| | 8 | ABC-123 | PC | 123-0001 | 8 | Day 1 | A554134-12 | DRGA_PAR | Drug A Parent | ANALYTE | PLASMA | 42.740 | ng/mL | 4.74 | 4.74 | ng/mL |
| 0.10 | 20 | 1 | DAY 1 | 1 | 2001-02-01T09:30 |
| 1 | 1H30MIN240M | 1.5 | Day 1 Dose | 2001-02-01T08:00 | PT1H30MPT240M |
| | 9 | ABC-123 | PC | 123-0001 | 9 | Day 1 | A554134-13 | DRGA_MET | Drug A Metabolite | ANALYTE | PLASMA | 5.44 | ng/mL | 5.44 | 5.44 | ng/mL |
| 0.10 | 20 | 1 | DAY 1 | 1 | 2001-02-01T14:00 |
| 1 | 6H | 6 | Day 1 Dose | 2001-02-01T08:00 | PT6H00M |
| | 10 | ABC-123 | PC | 123-0001 | 10 | Day 1 | A554134-13 | DRGA_PAR | Drug A Parent | ANALYTE | PLASMA | 1.09 | ng/mL | 1.09 | 1.09 | ng/mL |
| 0.10 | 20 | 1 | DAY 1 | 1 | 2001-02-01T14:00 |
| 1 | 6H | 6 | Day 1 Dose | 2001-02-01T08:00 | PT6H |
| | 11 | ABC-123 | PC | 123-0001 | 11 | Day 1 | A554134-14 | DRGA_MET | Drug A Metabolite | ANALYTE | PLASMA |
|
|
|
|
| NOT DONE |
| 20 | 2 | DAY 2 | 2 | 2001-02-02T08:00 |
| 2 | 24H | 24 | Day 1 Dose | 2001-02-01T08:00 | PT24H |
| | 12 | ABC-123 | PC | 123-0001 | 12 | Day 1 | A554134-14 | DRGA_PAR | Drug A Parent | ANALYTE | PLASMA | <0.1 | ng/mL | <0.1 |
| ng/mL |
| 0.10 | 20 | 2 | DAY 2 | 2 | 2001-02-02T08:00 |
| 2 | 24H | 24 | Day 1 Dose | 2001-02-01T08:00 | PT24H |
| | 13 | ABC-123 | PC | 123-0001 | 13 | Day 11 | A554134-15 | DRGA_MET | Drug A Metabolite | ANALYTE | PLASMA | 3.41 | ng/mL | 3.41 | 3.41 | ng/mL |
| 0.10 | 20 | 3 | DAY 11 | 11 | 2001-02-11T07:45 |
| 11 | PREDOSE | 0 | Day 11 Dose | 2001-02-11T08:00 | -PT15M |
| | 14 | ABC-123 | PC | 123-0001 | 14 | Day 11 | A554134-15 | DRGA_PAR | Drug A Parent | ANALYTE | PLASMA | <0.1 | ng/mL | <0.1 |
| ng/mL |
| 0.10 | 20 | 3 | DAY 11 | 11 | 2001-02-11T07:45 |
| 11 | PREDOSE | 0 | Day 11 Dose | 2001-02-11T08:00 | -PT15M |
| | 15 | ABC-123 | PC | 123-0001 | 15 | Day 11 | A554134-16 | DRGA_MET | Drug A Metabolite | ANALYTE | PLASMA | 8.74 | ng/mL | 8.74 | 8.74 | ng/mL |
| 0.10 | 20 | 3 | DAY 11 | 11 | 2001-02-11T09:30 |
| 11 | 1H30MIN | 1.5 | Day 11 Dose | 2001-02-11T08:00 | PT1H30M |
| | 16 | ABC-123 | PC | 123-0001 | 16 | Day 11 | A554134-16 | DRGA_PAR | Drug A Parent | ANALYTE | PLASMA | 4.2 | ng/mL | 4.2 | 4.2 | ng/mL |
| 0.10 | 20 | 3 | DAY 11 | 11 | 2001-02-11T09:30 |
| 11 | 1H30MIN | 1.5 | Day 11 Dose | 2001-02-11T08:00 | PT1H30M |
| | 17 | ABC-123 | PC | 123-0001 | 17 | Day 11 | A554134-17 | DRGA_MET | Drug A Metabolite | ANALYTE | URINE | 245 | ng/mL | 245 | 245 | ng/mL |
| 2.00 | 500 | 3 | DAY 11 | 11 | 2001-02-11T08:00 | 2001-02-11T14:03 | 11 | 6H | 6 | Day 11 Dose | 2001-02-11T08:00 | PT6H | -PT6H | | 18 | ABC-123 | PC | 123-0001 | 18 | Day 11 | A554134-17 | DRGA_PAR | Drug A Parent | ANALYTE | URINE | 13.1 | ng/mL | 13.1 | 13.1 | ng/mL |
| 2.00 | 500 | 3 | DAY 11 | 11 | 2001-02-11T08:00 | 2001-02-11T14:03 | 11 | 6H | 6 | Day 11 Dose | 2001-02-11T08:00 | PT6H | -PT6H | | 19 | ABC-123 | PC | 123-0001 | 19 | Day 11 | A554134-17 | VOLUME | Volume | SPECIMEN PROPERTY | URINE | 574 | mL | 574 | 574 | mL |
|
|
| 3 | DAY 11 | 11 | 2001-02-11T08:00 | 2001-02-11T14:03 | 11 | 6H | 6 | Day 11 Dose | 2001-02-11T08:00 | PT6H | -PT6H | | 20 | ABC-123 | PC | 123-0001 | 20 | Day 11 | A554134-17 | PH | PH | SPECIMEN PROPERTY | URINE | 5.5 |
| 5.5 | 5.5 |
|
|
|
| 3 | DAY 11 | 11 | 2001-02-11T08:00 | 2001-02-11T14:03 | 11 | 6H | 6 | Day 11 Dose | 2001-02-11T08:00 | PT6H | -PT6H | | 21 | ABC-123 | PC | 123-0001 | 21 | Day 11 | A554134-18 | DRGA_MET | Drug A Metabolite | ANALYTE | PLASMA | 9.02 | ng/mL | 9.02 | 9.02 | ng/mL |
| 0.10 | 20 | 3 | DAY 11 | 11 | 2001-02-11T14:00 |
| 11 | 6H | 6 | Day 11 Dose | 2001-02-11T08:00 | PT6H |
| | 22 | ABC-123 | PC | 123-0001 | 22 | Day 11 | A554134-18 | DRGA_PAR | Drug A Parent | ANALYTE | PLASMA | 1.18 | ng/mL | 1.18 | 1.18 | ng/mL |
| 0.10 | 20 | 3 | DAY 11 | 11 | 2001-02-11T14:00 |
| 11 | 6H | 6 | Day 11 Dose | 2001-02-11T08:00 | PT6H |
| | 23 | ABC-123 | PC | 123-0001 | 23 | Day 11 | A554134-19 | DRGA_MET | Drug A Metabolite | ANALYTE | URINE | 293 | ng/mL | 293 | 293 | ng/mL |
| 2.00 |
| 3 | DAY 11 | 11 | 2001-02-11T14:03 | 2001-02-11T20:10 | 11 | 12H | 12 | Day 11 Dose | 2001-02-11T08:00 | PT12H | -PT6H | | 24 | ABC-123 | PC | 123-0001 | 24 | Day 11 | A554134-19 | DRGA_PAR | Drug A Parent | ANALYTE | URINE | 7.1 | ng/mL | 7.1 | 7.1 | ng/mL |
| 2.00 |
| 3 | DAY 11 | 11 | 2001-02-11T14:03 | 2001-02-11T20:10 | 11 | 12H | 12 | Day 11 Dose | 2001-02-11T08:00 | PT12H | -PT6H | | 25 | ABC-123 | PC | 123-0001 | 25 | Day 11 | A554134-19 | VOLUME | Volume | SPECIMEN PROPERTY | URINE | 363 | mL | 363 | 363 | mL |
|
|
| 3 | DAY 11 | 11 | 2001-02-11T14:03 | 2001-02-11T20:10 | 11 | 12H | 12 | Day 11 Dose | 2001-02-11T08:00 | PT12H | -PT6H | | 26 | ABC-123 | PC | 123-0001 | 26 | Day 11 | A554134-19 | PH | PH | SPECIMEN PROPERTY | URINE | 5.5 |
| 5.5 | 5.5 |
|
|
|
| 3 | DAY 11 | 11 | 2001-02-11T14:03 | 2001-02-11T20:10 | 11 | 12H | 12 | Day 11 Dose | 2001-02-11T08:00 | PT12H | -PT6H | | 27 | ABC-123 | PC | 123-0001 | 27 | Day 11 | A554134-20 | DRGA_MET | Drug A Metabolite | ANALYTE | URINE | 280 | ng/mL | 280 | 280 | ng/mL |
| 2.00 |
| 4 | DAY 12 | 12 | 2001-02-11T20:03 | 2001-02-12T08:10 | 12 | 24H | 24 | Day 11 Dose | 2001-02-11T08:00 | PT24H | -PT12H | | 28 | ABC-123 | PC | 123-0001 | 28 | Day 11 | A554134-20 | DRGA_PAR | Drug A Parent | ANALYTE | URINE | 2.4 | ng/mL | 2.4 | 2.4 | ng/mL |
| 2.00 |
| 4 | DAY 12 | 12 | 2001-02-11T20:03 | 2001-02-12T08:10 | 12 | 24H | 24 | Day 11 Dose | 2001-02-11T08:00 | PT24H | -PT12H | | 29 | ABC-123 | PC | 123-0001 | 29 | Day 11 | A554134-20 | VOLUME | Volume | SPECIMEN PROPERTY | URINE | 606 | mL | 606 | 606 | mL |
|
|
| 4 | DAY 12 | 12 | 2001-02-11T20:03 | 2001-02-12T08:10 | 12 | 24H | 24 | Day 11 Dose | 2001-02-11T08:00 | PT24H | -PT12H | | 30 | ABC-123 | PC | 123-0001 | 30 | Day 11 | A554134-20 | PH | PH | SPECIMEN PROPERTY | URINE | 5.5 |
| 5.5 | 5.5 |
|
|
|
| 4 | DAY 12 | 12 | 2001-02-11T20:03 | 2001-02-12T08:10 | 12 | 24H | 24 | Day 11 Dose | 2001-02-11T08:00 | PT24H | -PT12H | | 31 | ABC-123 | PC | 123-0001 | 31 | Day 11 | A554134-21 | DRGA_MET | Drug A Metabolite | ANALYTE | PLASMA | 3.73 | ng/mL | 3.73 | 3.73 | ng/mL |
| 0.10 | 20 | 4 | DAY 12 | 12 | 2001-02-12T08:00 |
| 12 | 24H | 24 | Day 11 Dose | 2001-02-11T08:00 | PT24H |
| | 32 | ABC-123 | PC | 123-0001 | 32 | Day 11 | A554134-21 | DRGA_PAR | Drug A Parent | ANALYTE | PLASMA | <0.1 | ng/mL | <0.1 |
| ng/mL |
| 0.10 | 20 | 4 | DAY 12 | 12 | 2001-02-12T08:00 |
| 12 | 24H | 24 | Day 11 Dose | 2001-02-11T08:00 | PT24H |
| |
|
